Placing a coefficient of 2 before the product however will balance out the equation and is written as N 2 O 2 2NO. A reactant is a substance yond is presenteth at the starteth of a chemical reaction.

Chemical Reactions Balanced And Unbalanced Chemical Equations Online Science Notes
The purpose of a chemical equation is to depict or show a chemical reaction.
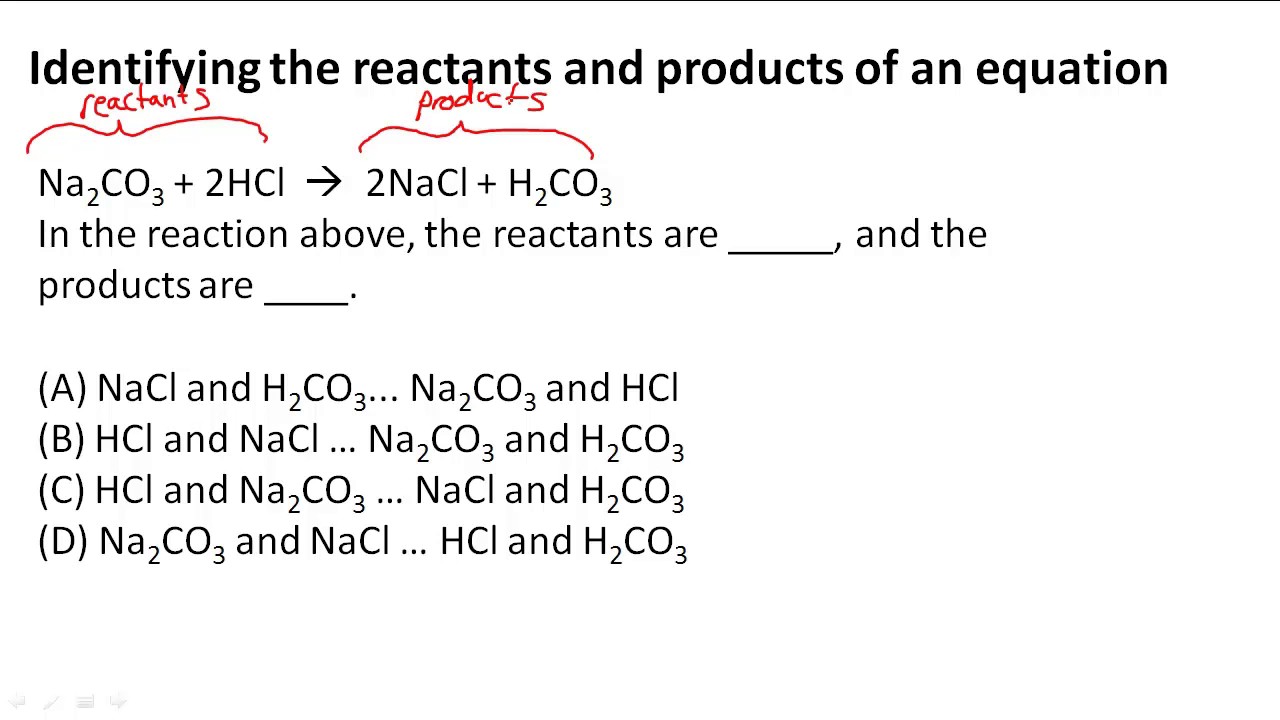
What is the reactant in a chemical equation. Methane combusts in oxygen to form carbon dioxide heat and water. Atomic symbols are used to represent the elements that take part in a reaction. A product is a substance yond is presenteth at the endeth of a chemical reaction So in this example 3CO g Fe2O3 s art the reactants.
H 2 hydrogen gas and O 2 oxygen gas are reactants in the reaction that forms liquid water. The balanced chemical equation for the reaction is. 411 2 H 2 O 2 2 H 2 O Chemical formulas and other symbols are used to indicate the starting materials or reactants which by convention are written on the left side of the equation and the final compounds or products which are written on the right.
4NH 3 5O 2 4NO 6H 2 O. A chemical equation is written with the reactants on the left side of an arrow and the products of the chemical reaction on the right. Classically chemical reactions encompass changes that only involve the positions of electrons in the forming and breaking of chemical bonds between atoms with no change to the nuclei no change to the elements present and can often be described by a chemical equation.
The nitrogen atoms and oxygen atoms on the left-hand reactant side of the equation do not equal the number of atoms on the right-hand product side. These ions are referred to as spectator ions because they do not participate in the chemical reaction. A chemical equation is the symbolic representation of a chemical reaction in the form of symbols and formulae wherein the reactant entities are given on the left-hand side and the product entities on the right-hand side.
Notice mass is conserved in this equation. For a closed system at constant volume this is often of the form For reactions that go to completion which implies very small k r or if only the initial rate is analyzed. The substances to the right of the arrow are called products.
The coefficients next to the symbols and formulae of entities are the absolute values of the stoichiometric numbersThe first chemical equation was diagrammed by Jean Beguin. Reactants are converted to products and the process is symbolized by a chemical equation. An arrow points from the reactant to the products.
A chemical reaction is a process that leads to the chemical transformation of one set of chemical substances to another. 311 2 H 2 O 2 2 H 2 O Chemical formulas and other symbols are used to indicate the starting materials or reactants which by convention are written on the left side of the equation and the final compounds or products which are written on the right. The numbers represent the ratios of reactants and products that produce the reaction.
To do so we simply divide the stoichiometric coefficients of each reactant. Updated December 23 2018 The limiting reactant or limiting reagent is a reactant in a chemical reaction that determines the amount of product that is formed. Chemical Reaction Formula A chemical reaction involves the rearranging of atoms of the same or different elements to form new substances.
The arrow signifies that the reaction forms or yields iron sulfide the product. CaCl 2 2AgNO 3 CaNO 3 2 2AgCl Ionic Equation. A reactantis a substance that is present at the start of a chemicalreaction.
2 H 2 g O 2 g 2 H 2 O l. There are four atoms of hydrogen in both the reactant and product side of the equation and two atoms of oxygen. For a chemical reaction a A b B p P q Q the rate equation or rate law is a mathematical expression used in chemical kinetics to link the rate of a reaction to the concentration of each reactant.
Identification of the limiting reactant makes it possible to calculate the theoretical yield of a reaction. The chemical equation for this reaction is written as. For example iron Fe and sulfur S combine to form iron sulfide FeS.
The substance s to the right of the arrow art hath called products. N 2 O 2 NO. Chemical equations are representations of chemical reactions in terms of symbols of elements and formulas of compounds.
A product is a substance that is present at the end of a chemicalreaction. Fe s S s FeS s The plus sign indicates that iron reacts with sulfur. Which chemicals react with one another These reacting chemicals are referred to as reactants Reactants are always displayed on the left of the reaction The resulting chemicals made from the reaction.
It is represented by a chemical equation in which the reactants substances that are broken apart are written on the left and the products new substances formed are written on the right. For example this is an unbalanced equation. The head of the arrow typically points toward the right or the product side of the equation although some equations may indicate equilibrium with the reaction proceeding in both directions simultaneously.
We start by finding the stoichiometric ratio of the reactants. Ca 2 2Cl 2Ag 2NO 3 Ca 2 2NO 3 2AgCl Comparing the reactants and the products of the ionic equation and the chemical equation it can be observed that the Ca 2 calcium ion and the NO 3 nitrate ions are present on both sides of the ionic equation. Reactants are the substances that enter inti a chemical reaction and the products are the substances formed.
The substances to the left of the arrow in a chemical equationare called reactants. The equation is read as 4 moles of ammonia require 5 moles of oxygen to produce 4 moles of nitric acid and 6 moles of water. In relation to my course teaching can change the lives of the students.
On the other hand a chemical equation is the symbolic representation of a chemical reaction. Gwendolyn Aceves _____ Chemical Equations and Reactions What is a Chemical Equation.
A simulation about reactants products and leftovers httpphetcoloradoeduensimulationreactants-products-and-leftovers. Reactants are consumed during the progression of a chemical reaction.
Sodium and chloride ions for example are the reactants in the production of table salt.
What are reactants in a chemical reaction. In a chemical reaction the atoms and molecules produced by the reaction are called products. Reactants are consumed in the reaction. The arrow is read as the word yields.
For example burning methane in oxygen is irreversible. Some chemical reactions go to completion resulting in all of the reactants becoming products. The properties of the products are different from those of the reactants.
Vinegar and baking soda are reactants when you mix them together they bubble up and make really good lava for a model volcano. The new substance that is formed is called the product of the reaction. No new atoms are created and no atoms are destroyed.
Instead you create a new substance with chemical reactions. Reactants are consumed in a chemical reaction. The active metals calcium and sodium both react with water to form hydrogen gas and a base.
Reactants are the starting material that undergoes changes during a chemical reaction. In a chemical formula the reactants are on the left side of the arrow and the products are on the right. Chemical Reactions and Chemical Equations The atoms and molecules that interact are called the reactants.
Necessity in Chemical Reactions. The atoms in the reactants have been rearranged into new compounds the products. Reactants are substances that start a chemical reaction.
In a chemical reaction substances elements andor compounds called reactants are changed into other substances compounds andor elements called products. A reactant is a substance that changes in a chemical reaction. This means that the total mass of the reactants.
During a chemical reaction the reactants are used to make the products. Substances are either chemical elements or compounds. The Chemical Nature of the Reacting Substances For example when small pieces of the metals iron and sodium are exposed to air the sodium reacts completely with air overnight whereas the iron is barely affected.
Reactants are substances initially present in a chemical reaction that are consumed during the reaction to make products. At the end of the reaction none of the reactants may be present in the reaction mixture but sometimes some of the reactants may be present at the end. The products are carbon dioxide gas and water vapor.
Other examples of everyday life chemical reactions include. Substances that chemically react with each other are called reagents and reactants. Reactants are chemical species that act as the starting material of a chemical reaction.
They consist of chemical or structural formulas of the reactants on the left and those of the products on the right. Catalysts work by increasing the frequency of collisions between reactants altering the orientation of reactants so that more collisions are effective reducing intramolecular bonding within reactant molecules or donating electron density to the reactants. Chemical equations are used to graphically illustrate chemical reactions.
Reactants are involved in all chemical reactions. The tip of the arrow points in the direction in which the reaction proceeds. Products are substances that are produced in the reaction.
The chemical bonds in reactant compounds are broken in order to form new bonds making a new compound. In a chemical reaction only the atoms present in the reactants can end up in the products. React together are called the reactants are formed in the reaction are called the products No atoms are created or destroyed in a chemical reaction.
This new compound is called the product of the reaction. The one or more substances produced by a chemical reaction are called the product. A chemical reaction rearranges the constituent atoms of the reactants to create different substances as products.
In a chemical reaction the reactants are the elements present when the reaction begins and the products are the elements and compounds produced as a result of the reaction. Rusting of iron burning of wood photosynthesis and combustion in the cars. You cant change one element into another in a chemical reaction that happens in nuclear reactions.
A chemical reaction is a process in which one or more substances also called reactants are converted to one or more different substances known as products. They are separated by an arrow which indicates the direction and type of the reaction. In other words not all chemical reactions necessarily required a chemical reagent.
A reaction can occur even without a chemical reagent. These reactions are said to be irreversible. The atoms and molecules produced by the reaction are called products.
In a chemical reaction the atoms and molecules that interact with each other are called reactants. All chemical reactions begin with a reactant the general term for the one or more substances that enter into the reaction. Catalysts eg enzymes lower the activation energy of a chemical reaction and increase the rate of a chemical reaction without being consumed in the process.
When a candle burns the reactants are fuel the candlewick and wax and oxygen in the air. A chemical reaction is often accompanied by a temperature change bubbles color change andor precipitate formation. It is a necessary component of a chemical reaction.