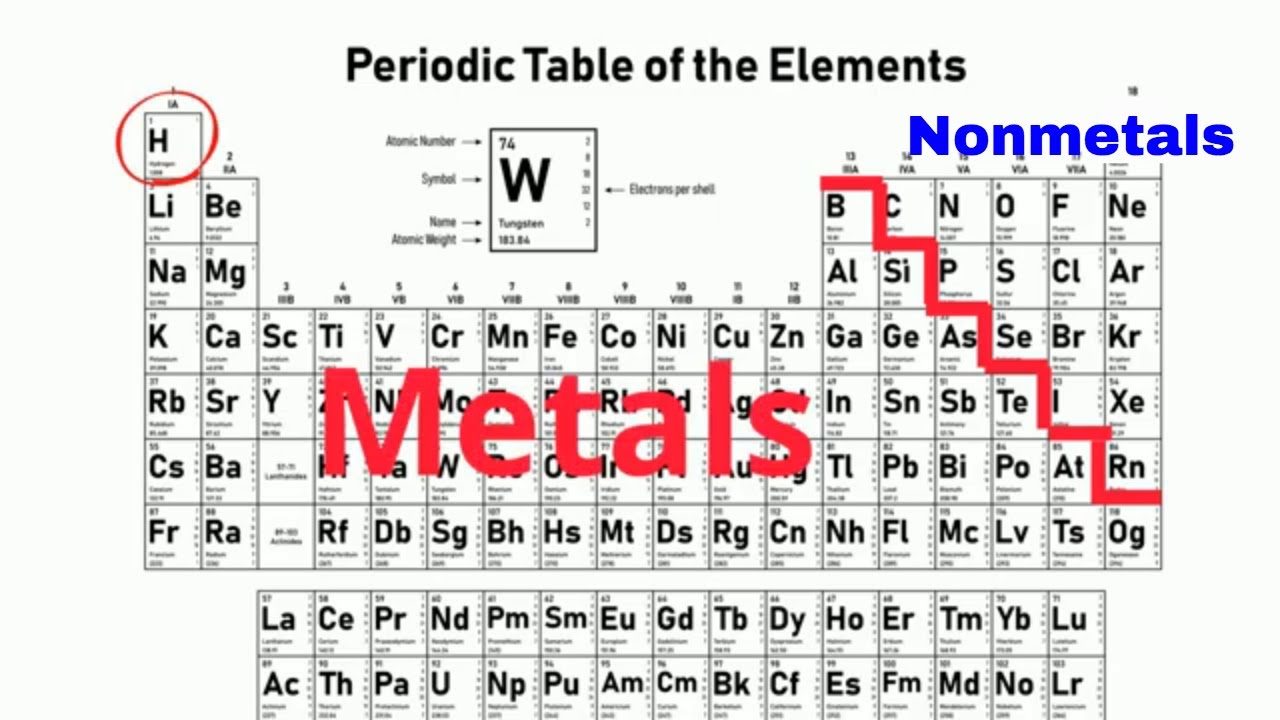
1 This group of elements is made up of all different states of matter solid liquid and gas a Nonmetals b Metals c Metalloids 2 These elements always have a shiny luster a Nonmetals b Metals c Metalloids 3 These elements are located in a stair step pattern on the periodic table a Nonmetals b Metals c Metalloids 4 Elements in this group are good conductors of heat AND electricity a Nonmetals b Metals c Metalloids 5 This tells you. Metals Nonmetals and Metalloids Using the periodic table you can classify the elements in many ways.
How To Identify Metals Nonmetals And Metalloids On The Periodic Table Youtube
Nonmetals are found in s and p blocks.

Periodic table with metals metalloids and nonmetals. Non-metals are grouped on the right side of the periodic table. These elements are called metalloids or. Metalloids are found in the middle of the periodic table.
Metals Nonmetals Metalloids - Maze chase. Your essay must have an introduction 1 paragraph a body 3 paragraphs and a conclusion 1 paragraph. Some elements between the metals and non-metals in the periodic table have properties which are a mixture of the properties of metals and non-metals.
Metals are found in the left side of the periodic table. Metals Metalloids and Nonmetals Essay Compare and contrast the three different types of elements using their physical properties and explain how you are able to determine if an element is a metal nonmetal or metalloid. This is an online quiz called Periodic Table Metals NonMetals and Metalloids There is a printable worksheet available for download here so you can take the quiz with pen and paper.
Elements are further classified into metals non-metals and metalloids based on their properties which are correlated with their placement in the periodic table. They form a separating boundary between the metals and nonmetals. Metalloids straddling the metal-nonmetal border are mostly distinct from either but in a few properties resemble one or the other as shown in the shading of the metalloid column below and summarized in the small table at the top of this section.
Position in the Periodic Table. According to their shared physical and chemical properties the elements can be classified into the major categories of metals metalloids and nonmetals. What is Carbon.
SOLIDS LIQUIDS or GASSES What makes up most of the periodic table What is the classification of these characteristics. Block in the Periodic Table. On a standard periodic table all eleven elements are in a diagonal region of the p-block extending from boron at the upper left to astatine at lower right.
The metals are to the left of the line except for hydrogen which is a nonmetal the nonmetals are to the right of the line and the elements immediately adjacent to the line are the metalloids. Some authors count metalloids as nonmetals with weakly nonmetallic properties. When elements combine to form compounds there are two major types of bonding that can result.
Metals are grouped on the left side of the periodic table with an exception of a hydrogen atom. The line begins at boron B and extends down to polonium Po. Search Help in Finding Periodic Table Metals NonMetals and Metalloids - Online Quiz Version Periodic Table Metals NonMetals and Metalloids online quiz.
Metals left side of a period generally have a lower electron affinity than nonmetals right side of a period with the exception of the noble gases. In other words metalloids semimetals are located on the right side of the post transition metals and on the left side of nonmetals see above image. Some periodic tables include a dividing line between metals and nonmetals and the metalloids may be found close to this line.
Also many periodic tables have a stair-step line on the table identifying the element groups. On many periodic tables a jagged black line see figure below along the right side of the table separates the metals from the nonmetals. Also include the location of the elements on the periodic table.
Where at the metalloids located on the Periodic Table. A description and practice of finding metals nonmetals and metalloids on the Periodic TableIn general metals are found on the left-hand side of the period. Elements of the periodic table are grouped as metals metalloids or semimetals and nonmetals.
Can a metalloid have properties of metals and nonmetals. One useful way is by metals nonmetals and metalloids. Nonmetals are found in the right side f the periodic table.
There are 118 elements known to us out of which 92 are naturally occurring while the rest have been prepared artificially. Where are metals located on the periodic table Most metals are. And metalloids are the borderline.
Authors differ in where they divide metals from nonmetals and in whether they recognize an intermediate metalloid category. Metalloids are located between the metals and nonmetals. The metals are found on the left and the nonmetals are found on the right.
The orange color on the Periodic table represents metalloids. The periodic table is organized in families and periods. Metals are located in s p d and f blocks.
The metalloids separate the metals and nonmetals on a periodic table. Learn about the metals nonmetals and metalloids and the periodic table.
A description and practice of finding metals nonmetals and metalloids on the Periodic TableIn general metals are found on the left-hand side of the period. The exception is the element hydrogen.
Most non-metals are brittle and are neither malleable nor ductile.

Periodic table showing metals nonmetals and semimetals. Metals and non-metals There are many divisions in the Periodic Table but one of the most important is between the metals and the non-metals. On The Periodic Table Elements Can Be Grouped Into The Metals Semimetals And The Non-metals Based On Their Properties. Periodic table showing metals nonmetals and semimetals.
It summarizes huge amounts of information about the elements in a way that facilitates the prediction of many of their properties and chemical reactions. They are usually solids or gases at room temperature with low melting and boiling points boron and carbon are exceptions. Metals nonmetals and metalloids make up the periodic table with metals constituting the large majority of all metals.
Atomic structure and the periodic table Elements in group 1 and group 2 are metals. Each element has a unique chemical symbol. Elements present to the left of the line of the periodic table are said as metals though elements present to the far right of the periodic table are said as non-metals.
Position of Nonmetals on the Periodic Table. State How These Properties Differ In Cach Elemental Category. Elements of the periodic table are grouped as metals metalloids or semimetals and nonmetals.
The semimetals lie along a diagonal line separating the metals and nonmetals. Metals are on the left of the periodic table and non-metals are on the right. The line begins at boron B and extends down to polonium Po.
The elements are arranged in a periodic table which is probably the single most important learning aid in chemistry. Elements to the lower left of the line generally display increasing metallic behaviour. Astatine atomic number 85 shows characteristics of nonmetals halogens as well as metalloids.
Semimetals or metalloids are found in a zig zag line on the periodic table separating the basic metals from. The only liquid non-metal is bromine. The reactive nonmetals near the metalloids show some incipient metallic character such as the metallic appearance of graphite black phosphorus selenium and iodine.
Elements to the left of the line are considered metals. The semimetals lie along a diagonal line separating the metals and nonmetals. Elements to the upper right display increasing nonmetallic behaviour.
Non-metals are found on the far right hand side of the table. There are 18 nonmetals on the Periodic table. An element can be identified in 3 different ways.
The metals are on the bottom left in the periodic table and the nonmetals are at the top right. The metal elements are on the left of a stepped line. Their shape can be easily changed into thin wires or sheets without breaking.
Metalloids or semimetals are present just to the right of metals and possess properties of metals as well as non-metals. In The Following Table Three Elemental Properties Are Listed. Number of metalloid properties that resemble metals or nonmetals or that.
The metals green in the table nonmetals orange and metalloids blue. Also many periodic tables have a stair-step line on the table identifying the element groups. All these nonmetals are located on the upper right corner of the Periodic table Hydrogen is located on the left top corner In the above image the nonmetals are represented in yellow color.
Metals are found on the left hand side of the table. An interactive Periodic table can be found here. The metalloids separate the metals and nonmetals on a periodic table.
The metals list which makes up the periodic table includes iron lead gold aluminum platinum uranium zinc lithium sodium tin silver etc. The Periodic Table contains a lot of useful information on the elements. The periodic table of metals and nonmetals can be broken down to give you a sense of each elements characteristics.
From left to right in the periodic table the nonmetals can be divided into the reactive nonmetals and the noble gases. Elements just to the right of the line exhibit properties of both metals and nonmetals and are termed metalloids or semimetals. The nonmetals lis t which makes up the periodic table includes hydrogen helium carbon sulfur nitrogen oxygen radon neon other halogens and noble gases etc.
Some nonmetals C black P S and Se are brittle solids at room temperature although each of these also have malleable pliable or ductile allotropes. Metals In the periodic table you can see a stair-stepped line starting at Boron B atomic number 5 and going all the way down to. Using the periodic table you can classify the elements in many ways.
Atoms of group 1 elements have one. Each element has a unique name. Metals nonmetals and metalloids make up the periodic table with metals constituting the large majority of all metals.
See The Periodic Table On The Back Of This Sheet. The dividing line between metals and nonmetals can be found in varying configurations on some representations of the periodic table of the elements see mini-example right. Nonmetals are present on the right hand side of the periodic table.
The elements are arranged in a periodic table which is probably the single most important learning aid in chemistry. It summarizes huge amounts of information about the elements in a way that facilitates the prediction of many of their properties and chemical reactions. Semi-metals are found in the region between the metals and non-metals.
Look These Up In Your Text. One useful way is by metals nonmetals and metalloids. The periodic table is organized in families and periods.
