Elements within a group share several common properties and often have the same outer electron arrangement. They are sometimes called heavy metals and are denser than alkali or alkaline earth metals.
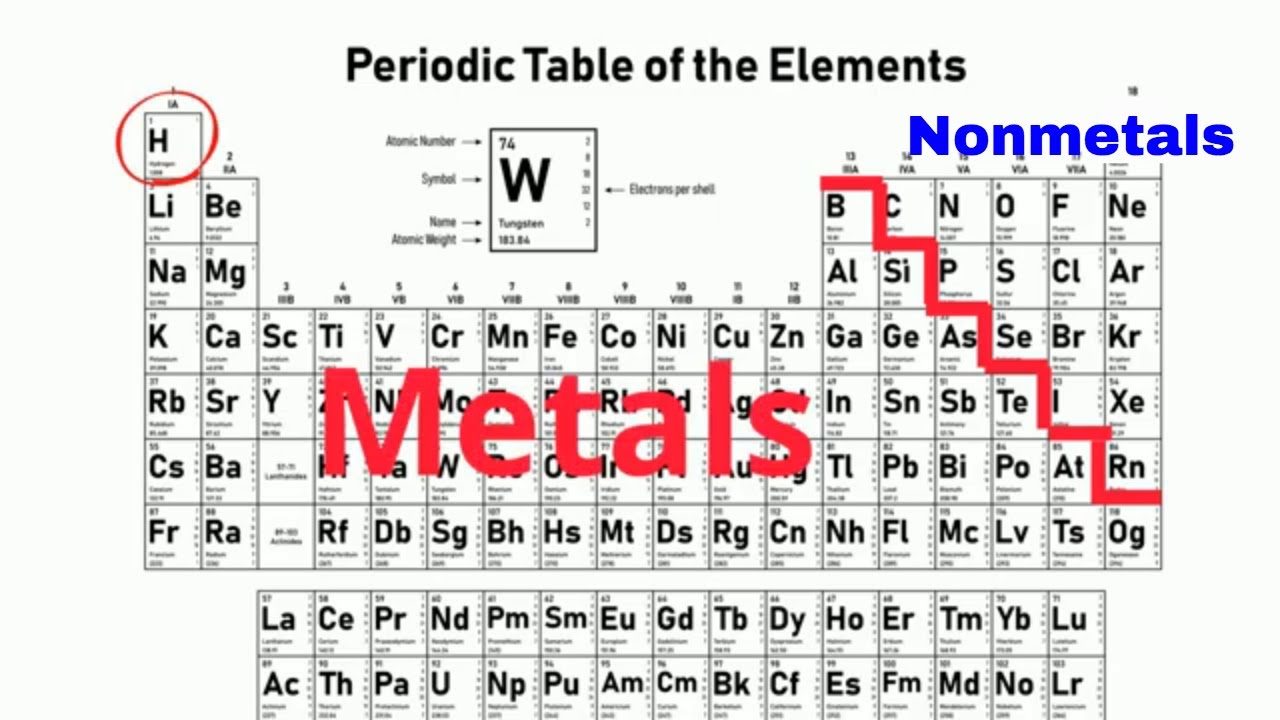
Except for Germanium Ge and Antimony Sb all the elements to the left of that line can be classified as metals.
The metals of the periodic table. The general features of the long form periodic table are. Get essential facts about the first 20 elements all in one convenient place including the name atomic number atomic mass element symbol group and electron configurationIf you need detailed facts about these elements or any of the higher numbered ones start with the clickable periodic table. The archetypal transition metals and the physically and chemically weak post-transition metals.
The transition metals are found in the center of the main body of the Periodic Table. Periodic table in chemistry the organized array of all the chemical elements in order of increasing atomic number. Other resources related to the Periodic Table.
Here is a Periodic Table of elements with everything you need to know. Metals In The Periodic Table. So because most elements of the Table are metals it makes sense to begin by looking at them.
From left to right in the periodic table these categories include the highly reactive alkali metals. Interactive periodic table with up-to-date element property data collected from authoritative sources. Click on any elements name for further chemical properties environmental data or health effects.
In the long form periodic table the elements are arranged in the order of their atomic numbers. If you look at the Periodic table you will find that the metal elements are located between atomic number 5 Boron B all the way to atomic number 84 Polonium Po. 93 Np Neptunium 237 94 Pu Plutonium 244 95 Am Americium 243 96 Cm Curium 247.
The metals consist of the alkali metals alkaline earths transition metals lanthanides and actinides. Chemical Evolution of the Universe. Metals reside on the left side of the table while non-metals reside on the right.
Elements that have similar properties are arranged in groups or families - vertical columns. 92 U Uranium 23803. Most elements can be considered metals.
Look up chemical element names symbols atomic masses and other properties visualize trends or even test your elements knowledge by playing a periodic table game. The periodic table also known as the periodic table of elements is organized so scientists can quickly discern the properties of individual elements such as their mass electron number electron configuration and their unique chemical properties. Elements are arranged in order of increasing atomic number their physical and chemical properties show a periodic pattern.
IE decreases going down a column of the periodic table and increases from left to right in a row. Most of the elements on the periodic table are metals. They are grouped together in the middle to the left-hand side of the periodic table.
This list contains the 118 elements of chemistry. List of Periodic Table Elements in Hebrew. The present periodic table has room for 118 elements.
The modern periodic table is based on the modern periodic law put forward by the English physicist Henry Moseley which states that the properties of. There are 38 transition metals including. The periodic table also known as the periodic table of elements is a tabular display of the chemical elements which are arranged by atomic number electron configuration and recurring chemical propertiesThe structure of the table shows periodic trendsThe seven rows of the table called periods generally have metals on the left and nonmetals on the right.
The chemical elements of the periodic chart sorted by. Atomic number of an element is equal to the number of protons inside the nucleus of its atom. In the periodic table you can see a stair-stepped line starting at Boron B atomic number 5 and going all the way down to Polonium Po atomic number 84.
Compared to other metals they are soft and have low melting points and densities. In the periodic table the vertical columns are called groups and the horizontal rows are called periods. The less reactive alkaline earth metals lanthanides and radioactive actinides.
Group 1 with one valence electron and Group 2 with two valence electrons are called the alkali metalsalkali metals metals found in Group 1 of the periodic table. It is arranged according to the periodic law. Youll get labeled Periodic table with Name Atomic mass Electron configuration Electronegativity Ionization energy Electron affinity etc.
The alkali metals alkaline earths basic metals transition metals lanthanides and actinides all are groups of metals. 91 Pa Protactinium 23104. The periodic table is the tabular arrangement of all the chemical elements on the basis of their respective atomic numbers.
When the elements are thus arranged there is a recurring pattern called the periodic law in their properties in which elements in the same column group have similar properties. Metals comprise the large majority of the elements and can be subdivided into several different categories. Our current periodic table lists 109 elements.
The horizontal rows are called. There are in all 18 vertical columns and 18 groups in the long form periodic table. Alkali metals are powerful reducing agents and form univalent compounds.
Thus alkali metals have the lowest IE in a period and Rare gases have the highest. The elements of the periodic table sorted by name in an alphabetical list. 89 Ac Actinium 227 90 Th Thorium 23204.
Properties of Metalloids Metalloids are malleable and ductile Families Families in the periodic table share chemical properties because all elements in a family have the same number of valence electrons This means that all elements in a. Metals are found in the left side of the periodic table.
Metals Nonmetals And Metalloids Mooramo
Position in the Periodic Table.

The periodic table metals nonmetals and metalloids. Using it you should be able to classify all the elements in different ways. Development of the Periodic table Effective Nuclear Charge Atomic and Ionic sizes. The Periodic Table contains a lot of useful information on the elements.
One useful way is by metals nonmetals and metalloids. Metals nonmetals and metalloids worksheet pdf. From left to right in the periodic table the nonmetals can be divided into the reactive nonmetals and the noble gases.
The periodic table is organized in families and periods. Metalloids are elements which show properties intermediate between metals and nonmetalsMost of them have metallic lustre but are brittle like non metalsSome of them are ductileThey dont transmit electricity at room temperatures but when heated they act as conductorsThey are placed between metals and non metals in the periodic table. The metals are to the left of the line except for hydrogen which is a nonmetal the nonmetals are to the right of the line and the elements immediately adjacent to the line are the metalloids.
The periodic table on the left separates elements into three groups. Elements of the periodic table are grouped as metals metalloids or semimetals and nonmetals. Metals are located in s p d and f blocks.
Metals non metals metalloids please list at least 4 physical properties of metals non metals and metalloids. Metals and nonmetals author. The reactive nonmetals near the metalloids show some incipient metallic character such as the metallic appearance of graphite black phosphorus selenium and iodine.
Block in the Periodic Table. These are elements found on the left side of the periodic table and they tend to exhibit metallic behaviour. Identify the following as metals nonmetals or metalloids using the periodic table.
Metals are placed on the left side of the periodic table Non-metals are placed on the right side of the periodic table and Metalloids are placed in the middle of the periodic table. The noble gases are almost completely inert. Metals Nonmetals and Metalloids Using the periodic table you can classify the elements in many ways.
An Introduction to General Organic and Biological Chemistry 12th. Their shape can be easily changed into thin wires or sheets without breaking. Nonmetals are present on the right hand side of the periodic table.
They are usually solids or gases at room temperature with low melting and boiling points boron and carbon are exceptions. Most non-metals are brittle and are neither malleable nor ductile. The metals green in the table nonmetals orange and metalloids blue.
The line begins at boron B and extends down to polonium Po. Metals have a shiny appearance non-metals have a dull appearance. Metals are located in s p d and f blocks in the periodic table though non-metals is located in s and p blocks and metalloids are located in p block of the periodic table.
Metals Nonmetals and Metalloids Chemistry 101 Periodic Table properties. The only liquid non-metal is bromine. These elements are called metalloids or.
Metalloids are found in the middle of the periodic table. Nonmetals are found in s and p blocks. Properties of Metals Nonmetals Metalloids Created Date.
One of the best ways to classify the elements is into metals and non-metals. Some elements between the metals and non-metals in the periodic table have properties which are a mixture of the properties of metals and non-metals. Nonmetals are found in the right side f the periodic table.
Metals nonmetals and metalloids make up the periodic table with metals constituting the large majority of all metals. 9182015 24543 PM. 1 This group of elements is made up of all different states of matter solid liquid and gas a Nonmetals b Metals c Metalloids 2 These elements always have a shiny luster a Nonmetals b Metals c Metalloids 3 These elements are located in a stair step pattern on the periodic table a Nonmetals b Metals c Metalloids 4 Elements in this group are good conductors of heat AND.
3 DIFFERENT TYPES OF ELEMENTS. Periodic Table of the Elements Mg meta loids. However metalloids have a shiny and dull appearance.
Also many periodic tables have a stair-step line on the table identifying the element groups. Most elements are metalsThey are usually shiny very dense and only melt at high temperatures. They are found in a stair step line that helps differentiate metals from non-metals in this element table.
Metals are classified as basic metals alkali metals transition metals alkaline earth metals lanthanides and actinides. Properties of Metalloids They conduct electricity and heat better than nonmetals but not as well as metals. The metalloids also known as semi-metals are placed between metals and non-metals in the periodic table of elements.
METALS NONMETALS METALLOIDS Classifying elements on the Periodic Table. There are seven elements that are classified as metalloids and placed in Group 13 14 15 16 and 17. 12 12 2013 12 07 59 pm.
The metalloids separate the metals and nonmetals on a periodic table. On many periodic tables a jagged black line see figure below along the right side of the table separates the metals from the nonmetals.
A description and practice of finding metals nonmetals and metalloids on the Periodic TableIn general metals are found on the left-hand side of the period. The exception is the element hydrogen.
Most non-metals are brittle and are neither malleable nor ductile.

Periodic table showing metals nonmetals and semimetals. Metals and non-metals There are many divisions in the Periodic Table but one of the most important is between the metals and the non-metals. On The Periodic Table Elements Can Be Grouped Into The Metals Semimetals And The Non-metals Based On Their Properties. Periodic table showing metals nonmetals and semimetals.
It summarizes huge amounts of information about the elements in a way that facilitates the prediction of many of their properties and chemical reactions. They are usually solids or gases at room temperature with low melting and boiling points boron and carbon are exceptions. Metals nonmetals and metalloids make up the periodic table with metals constituting the large majority of all metals.
Atomic structure and the periodic table Elements in group 1 and group 2 are metals. Each element has a unique chemical symbol. Elements present to the left of the line of the periodic table are said as metals though elements present to the far right of the periodic table are said as non-metals.
Position of Nonmetals on the Periodic Table. State How These Properties Differ In Cach Elemental Category. Elements of the periodic table are grouped as metals metalloids or semimetals and nonmetals.
The semimetals lie along a diagonal line separating the metals and nonmetals. Metals are on the left of the periodic table and non-metals are on the right. The line begins at boron B and extends down to polonium Po.
The elements are arranged in a periodic table which is probably the single most important learning aid in chemistry. Elements to the lower left of the line generally display increasing metallic behaviour. Astatine atomic number 85 shows characteristics of nonmetals halogens as well as metalloids.
Semimetals or metalloids are found in a zig zag line on the periodic table separating the basic metals from. The only liquid non-metal is bromine. The reactive nonmetals near the metalloids show some incipient metallic character such as the metallic appearance of graphite black phosphorus selenium and iodine.
Elements to the left of the line are considered metals. The semimetals lie along a diagonal line separating the metals and nonmetals. Elements to the upper right display increasing nonmetallic behaviour.
Non-metals are found on the far right hand side of the table. There are 18 nonmetals on the Periodic table. An element can be identified in 3 different ways.
The metals are on the bottom left in the periodic table and the nonmetals are at the top right. The metal elements are on the left of a stepped line. Their shape can be easily changed into thin wires or sheets without breaking.
Metalloids or semimetals are present just to the right of metals and possess properties of metals as well as non-metals. In The Following Table Three Elemental Properties Are Listed. Number of metalloid properties that resemble metals or nonmetals or that.
The metals green in the table nonmetals orange and metalloids blue. Also many periodic tables have a stair-step line on the table identifying the element groups. All these nonmetals are located on the upper right corner of the Periodic table Hydrogen is located on the left top corner In the above image the nonmetals are represented in yellow color.
Metals are found on the left hand side of the table. An interactive Periodic table can be found here. The metalloids separate the metals and nonmetals on a periodic table.
The metals list which makes up the periodic table includes iron lead gold aluminum platinum uranium zinc lithium sodium tin silver etc. The Periodic Table contains a lot of useful information on the elements. The periodic table of metals and nonmetals can be broken down to give you a sense of each elements characteristics.
From left to right in the periodic table the nonmetals can be divided into the reactive nonmetals and the noble gases. Elements just to the right of the line exhibit properties of both metals and nonmetals and are termed metalloids or semimetals. The nonmetals lis t which makes up the periodic table includes hydrogen helium carbon sulfur nitrogen oxygen radon neon other halogens and noble gases etc.
Some nonmetals C black P S and Se are brittle solids at room temperature although each of these also have malleable pliable or ductile allotropes. Metals In the periodic table you can see a stair-stepped line starting at Boron B atomic number 5 and going all the way down to. Using the periodic table you can classify the elements in many ways.
Atoms of group 1 elements have one. Each element has a unique name. Metals nonmetals and metalloids make up the periodic table with metals constituting the large majority of all metals.
See The Periodic Table On The Back Of This Sheet. The dividing line between metals and nonmetals can be found in varying configurations on some representations of the periodic table of the elements see mini-example right. Nonmetals are present on the right hand side of the periodic table.
The elements are arranged in a periodic table which is probably the single most important learning aid in chemistry. It summarizes huge amounts of information about the elements in a way that facilitates the prediction of many of their properties and chemical reactions. Semi-metals are found in the region between the metals and non-metals.
Look These Up In Your Text. One useful way is by metals nonmetals and metalloids. The periodic table is organized in families and periods.
Elements to the left of the line are considered metals. This line is often referred to as the staircase because of its shape.
Classification Of Elements In The Periodic Table Color Coding Yellow Download Scientific Diagram
The alkaline earth metals are found in column 2 on the left side of the Periodic Table.

Where are the metals on the periodic table. These are the representative elements or main group elements. This periodic table groups elements according to type. All of the metals are grouped.
There are only two exceptions ie two elements in that sequence between number 5 and number 84 that are not metals. The majority of elements on the periodic table are metals. In the periodic table the vertical columns are called groups and the horizontal rows are called periods.
In the periodic table you can see a stair-stepped line starting at Boron B atomic number 5 and going all the way down to Polonium Po atomic number 84. Also many periodic tables have a stair-step line on the table identifying the element groups. Periodic table in chemistry the organized array of all the chemical elements in order of increasing atomic number.
An elements position on the Periodic Table tells us whether it is a metal a non-metal or a semi-metal. The metalloids separate the metals and nonmetals on a periodic table. The archetypal transition metals and the physically and chemically weak post-transition metals.
Except for Germanium Ge and Antimony Sb all the elements to the left of that line can be classified as metals. H in the top left hand corner. The less reactive alkaline earth metals lanthanides and radioactive actinides.
The first 94 elements of the periodic table are naturally occurring while the rest from 95 to 118 have only been synthesized in laboratories or nuclear reactors. When the elements are thus arranged there is a recurring pattern called the periodic law in their properties in which elements in the same column group have similar properties. Most periodic tables print a thick black line to show the division between metals and nonmetals.
The periodic table is the tabular arrangement of all the chemical elements on the basis of their respective atomic numbers. The highlighted elements are considered the metal elements. Look up chemical element names symbols atomic masses and other properties visualize trends or even test your elements knowledge by playing a periodic table game.
The six alkaline earth metals are. The periodic table also known as the periodic table of elements is a tabular display of the chemical elements which are arranged by atomic number electron configuration and recurring chemical propertiesThe structure of the table shows periodic trendsThe seven rows of the table called periods generally have metals on the left and nonmetals on the right. If you look at the Periodic table you will find that the metal elements are located between atomic number 5 Boron B all the way to atomic number 84 Polonium Po.
Interactive periodic table with up-to-date element property data collected from authoritative sources. The line begins at boron B and extends down to polonium Po. Metals are found on the left hand side of the table.
The elements are arranged in order of increasing atomic number with the lightest element hydrogen. And atomic number 52 Antinomy Sb. American Elements is a US.
Metal blue nonmetal yellow or metalloid red. Atomic number 32 Germanium Ge. They are grouped together in the middle to the left-hand side of the periodic table.
Each element has a fixed position on the Periodic Table. Periodic table of the elements materials science and academic information elements and advanced materials data scientific presentations and all pages designs concepts logos and color schemes herein are the copyrighted proprietary rights and intellectual property of American Elements. The metals consist of the alkali metals alkaline earths transition metals lanthanides and actinides.
The modern periodic table is based on the modern periodic law put forward by the English physicist Henry Moseley which states that the properties of. The periodic table also known as the periodic table of elements is organized so scientists can quickly discern the properties of individual elements such as their mass electron number electron configuration and their unique chemical properties. F-block is located below the main table These are the inner transition metals or rare earth elements.
Most elements can be considered metals. Metals reside on the left side of the table while non-metals reside on the right. Get essential facts about the first 20 elements all in one convenient place including the name atomic number atomic mass element symbol group and electron configurationIf you need detailed facts about these elements or any of the higher numbered ones start with the clickable periodic table.
The modern periodic table the one we use now is a new and improved version of certain models put forth by scientists in the 19th and 20th century. Metals comprise the large majority of the elements and can be subdivided into several different categories. From left to right in the periodic table these categories include the highly reactive alkali metals.
They are generally harder and denser than alkali metals have 2 electrons in their outermost s sub-shell and each make a distinct color in their flames. D-block is group 3B to 2B look at the periodic table above because the numbers are not sequential These are the transition metals.
ads
Pages
Search This Blog
Blog Archive
- January 2023 (12)
- June 2022 (1)
- April 2022 (1)
- March 2022 (7)
- February 2022 (7)
- January 2022 (4)
- December 2021 (4)
- November 2021 (4)
- October 2021 (8)
- September 2021 (5)
- August 2021 (5)
- July 2021 (5)
- June 2021 (3)
- May 2021 (3)
- April 2021 (10)
- March 2021 (8)
- February 2021 (2)
- January 2021 (6)
- December 2020 (9)
- November 2020 (6)
- October 2020 (2)
- September 2020 (83)
- August 2020 (4)
- July 2020 (4)
- June 2020 (1)
- May 2020 (4)
- April 2020 (1)
- March 2020 (7)
- February 2020 (2)
- January 2020 (5)
- December 2019 (4)
- November 2019 (5)
- October 2019 (4)
- September 2019 (4)
- August 2019 (9)
- July 2019 (8)
- June 2019 (5)
- May 2019 (3)
- April 2019 (6)
- March 2019 (4)
- February 2019 (3)
- January 2019 (9)
- December 2018 (5)
- November 2018 (5)
- October 2018 (3)
- September 2018 (4)
- August 2018 (3)
- July 2018 (2)
Labels
- 1976
- 2010
- 2020
- access
- according
- accounting
- accounts
- accurate
- additionally
- administration
- adrenal
- advantages
- agenda
- alexander
- algae
- alpha
- alphabet
- alternative
- always
- amendment
- american
- analytics
- anatomy
- anchored
- android
- another
- answers
- aperture
- applying
- appraisal
- assets
- atom
- authorization
- baking
- balance
- balanced
- balls
- banking
- bankruptcy
- baseball
- based
- basic
- beginners
- beginning
- behaviorally
- benefits
- between
- bible
- bill
- blocks
- blood
- bookkeeping
- borax
- breaker
- building
- burn
- business
- buying
- cabinet
- calculate
- capital
- carbohydrate
- catch
- celiac
- cell
- certify
- chain
- change
- charging
- cheat
- checklist
- chemical
- chickens
- children
- childrens
- choose
- christmas
- church
- circular
- claims
- classroom
- clogged
- coaching
- coding
- commandments
- comments
- commons
- company
- competence
- complaint
- complex
- components
- comprehension
- concert
- confirmed
- congruent
- constitution
- control
- converter
- corporation
- cost
- crash
- cremated
- criminological
- criteria
- customer
- cycle
- data
- death
- define
- defined
- defrost
- delta
- denominations
- department
- depression
- derivation
- description
- descriptive
- desiderata
- desirable
- developed
- development
- diabetes
- diagonals
- difference
- differences
- diligence
- disadvantages
- discuss
- disk
- does
- dollar
- doric
- double
- dresses
- dummies
- effective
- elasticity
- electrical
- electronegativity
- electronic
- electronics
- elizabeth
- employed
- employee
- employees
- encouraging
- endosymbiosis
- engine
- engineering
- english
- entities
- entry
- equation
- equivalent
- erikson
- ernest
- estate
- euclidean
- euthanize
- exam
- example
- examples
- excel
- exchange
- exemplary
- existence
- expense
- experiences
- fashioned
- fatigue
- feed
- film
- finance
- find
- finishes
- fish
- floating
- flow
- fluid
- following
- force
- form
- format
- formula
- fraction
- from
- funny
- galactose
- galaxy
- gardner
- generation
- girdle
- glucose
- goddesses
- gods
- goes
- golden
- grade
- grammar
- grantor
- grasses
- gravitation
- great
- greek
- group
- guide
- happens
- hardship
- headband
- henley
- hero
- hidden
- hierarchy
- hindsight
- history
- holy
- honor
- horse
- howard
- improvement
- indirect
- info
- ingles
- inheriting
- insurance
- intangible
- intellectual
- inventory
- investment
- involvement
- ionic
- iron
- italian
- items
- jason
- johari
- journal
- judge
- july
- killed
- knit
- knowledge
- kubler
- laptop
- latex
- latin
- layout
- leadership
- leak
- leaking
- lean
- learning
- lease
- letter
- letterhead
- license
- located
- logarithmic
- lowered
- macro
- macromolecule
- magnetic
- magnuson
- management
- managerial
- manufacturing
- many
- marilyn
- marine
- market
- marketable
- marks
- maslow
- master
- math
- meaning
- measuring
- medical
- meeting
- metalloids
- metals
- method
- microsoft
- milian
- milk
- minecraft
- minorities
- missouri
- model
- modeling
- monthly
- motivation
- multiple
- multiples
- naughty
- needs
- nonmetals
- northern
- notice
- nuclear
- null
- number
- object
- objectives
- officers
- ofthe
- olympic
- operating
- organisms
- organization
- other
- overheats
- oxley
- oysters
- page
- panes
- parliamentary
- part
- particles
- parts
- passengers
- password
- pearls
- penny
- pentatonic
- percent
- percentile
- performance
- periodic
- permissions
- personality
- phases
- philosophy
- phrase
- phrases
- physics
- physiology
- plate
- plato
- player
- poema
- point
- pool
- portfolios
- powershell
- practices
- predictive
- pregnenolone
- president
- problems
- process
- product
- project
- prokaryotic
- pronoun
- pronunciation
- properly
- properties
- proprietorship
- protect
- publisher
- purgatory
- quadrilateral
- questions
- quick
- quiz
- quizzes
- quotation
- raising
- rank
- reactants
- reaction
- reading
- real
- recorder
- reference
- refracting
- refrigerator
- relationship
- renewal
- repeating
- report
- resignation
- response
- restore
- resume
- review
- right
- roberts
- role
- roles
- rule
- running
- sainthood
- sales
- sample
- sarbanes
- saving
- scale
- scales
- schedule
- science
- score
- scorecard
- scottish
- script
- season
- securities
- selling
- semimetals
- server
- service
- sharepoint
- sheet
- should
- shoulder
- shower
- shutter
- sigma
- silent
- simple
- single
- skill
- slime
- socrates
- softball
- solder
- soldering
- sole
- something
- song
- southern
- spanish
- specification
- speech
- speed
- speeds
- stage
- stages
- start
- starting
- statement
- states
- sticker
- stocks
- storage
- stories
- storm
- story
- storyboard
- student
- style
- subatomic
- swimming
- symptoms
- table
- tanks
- taxes
- tchaikovsky
- teachers
- team
- telescope
- tell
- temperature
- template
- termination
- terms
- tether
- texting
- theory
- thinner
- titanic
- tools
- toshiba
- transfer
- transistor
- transmission
- triangle
- trick
- trim
- trivia
- trust
- type
- types
- understanding
- uneven
- united
- universal
- unknown
- values
- volume
- voorhees
- wallpaper
- warm
- water
- wedding
- what
- whats
- when
- where
- which
- william
- window
- windows
- with
- without
- wizard
- words
- working
- write
- writing
- your
- youth
