Placing a coefficient of 2 before the product however will balance out the equation and is written as N 2 O 2 2NO. A reactant is a substance yond is presenteth at the starteth of a chemical reaction.

Chemical Reactions Balanced And Unbalanced Chemical Equations Online Science Notes
The purpose of a chemical equation is to depict or show a chemical reaction.
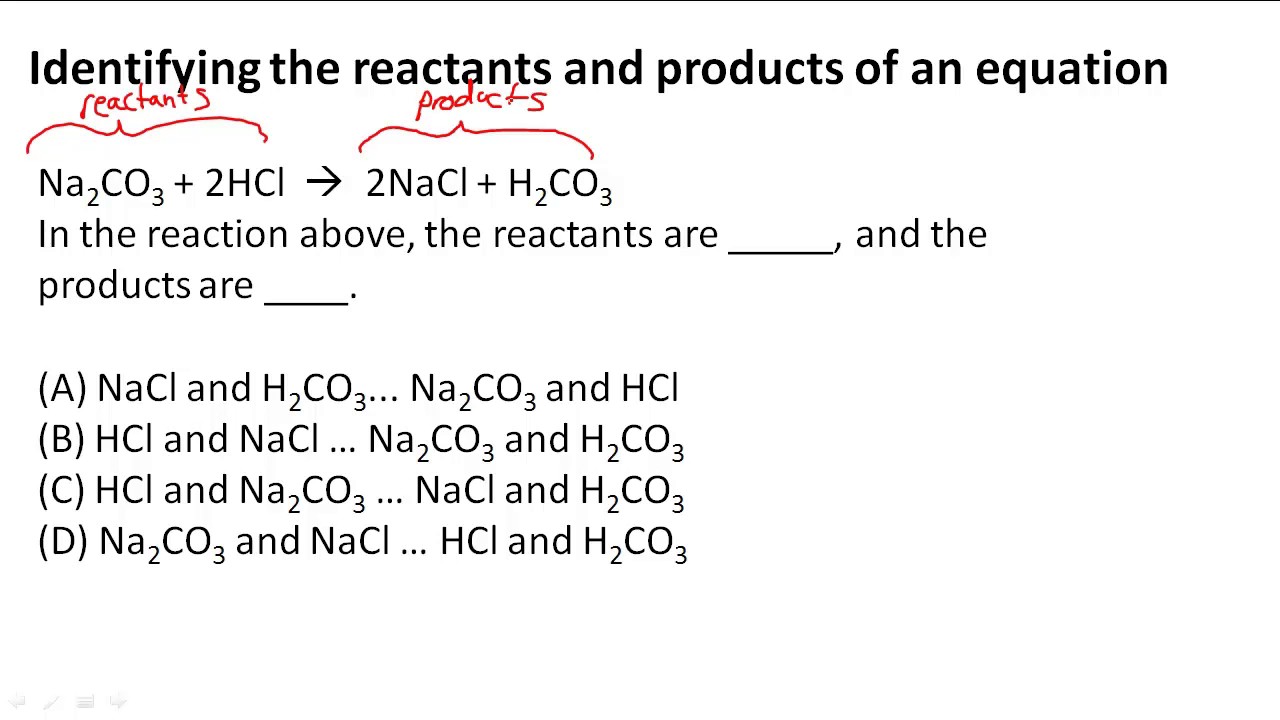
What is the reactant in a chemical equation. Methane combusts in oxygen to form carbon dioxide heat and water. Atomic symbols are used to represent the elements that take part in a reaction. A product is a substance yond is presenteth at the endeth of a chemical reaction So in this example 3CO g Fe2O3 s art the reactants.
H 2 hydrogen gas and O 2 oxygen gas are reactants in the reaction that forms liquid water. The balanced chemical equation for the reaction is. 411 2 H 2 O 2 2 H 2 O Chemical formulas and other symbols are used to indicate the starting materials or reactants which by convention are written on the left side of the equation and the final compounds or products which are written on the right.
4NH 3 5O 2 4NO 6H 2 O. A chemical equation is written with the reactants on the left side of an arrow and the products of the chemical reaction on the right. Classically chemical reactions encompass changes that only involve the positions of electrons in the forming and breaking of chemical bonds between atoms with no change to the nuclei no change to the elements present and can often be described by a chemical equation.
The nitrogen atoms and oxygen atoms on the left-hand reactant side of the equation do not equal the number of atoms on the right-hand product side. These ions are referred to as spectator ions because they do not participate in the chemical reaction. A chemical equation is the symbolic representation of a chemical reaction in the form of symbols and formulae wherein the reactant entities are given on the left-hand side and the product entities on the right-hand side.
Notice mass is conserved in this equation. For a closed system at constant volume this is often of the form For reactions that go to completion which implies very small k r or if only the initial rate is analyzed. The substances to the right of the arrow are called products.
The coefficients next to the symbols and formulae of entities are the absolute values of the stoichiometric numbersThe first chemical equation was diagrammed by Jean Beguin. Reactants are converted to products and the process is symbolized by a chemical equation. An arrow points from the reactant to the products.
A chemical reaction is a process that leads to the chemical transformation of one set of chemical substances to another. 311 2 H 2 O 2 2 H 2 O Chemical formulas and other symbols are used to indicate the starting materials or reactants which by convention are written on the left side of the equation and the final compounds or products which are written on the right. The numbers represent the ratios of reactants and products that produce the reaction.
To do so we simply divide the stoichiometric coefficients of each reactant. Updated December 23 2018 The limiting reactant or limiting reagent is a reactant in a chemical reaction that determines the amount of product that is formed. Chemical Reaction Formula A chemical reaction involves the rearranging of atoms of the same or different elements to form new substances.
The arrow signifies that the reaction forms or yields iron sulfide the product. CaCl 2 2AgNO 3 CaNO 3 2 2AgCl Ionic Equation. A reactantis a substance that is present at the start of a chemicalreaction.
2 H 2 g O 2 g 2 H 2 O l. There are four atoms of hydrogen in both the reactant and product side of the equation and two atoms of oxygen. For a chemical reaction a A b B p P q Q the rate equation or rate law is a mathematical expression used in chemical kinetics to link the rate of a reaction to the concentration of each reactant.
Identification of the limiting reactant makes it possible to calculate the theoretical yield of a reaction. The chemical equation for this reaction is written as. For example iron Fe and sulfur S combine to form iron sulfide FeS.
The substance s to the right of the arrow art hath called products. N 2 O 2 NO. Chemical equations are representations of chemical reactions in terms of symbols of elements and formulas of compounds.
A product is a substance that is present at the end of a chemicalreaction. Fe s S s FeS s The plus sign indicates that iron reacts with sulfur. Which chemicals react with one another These reacting chemicals are referred to as reactants Reactants are always displayed on the left of the reaction The resulting chemicals made from the reaction.
It is represented by a chemical equation in which the reactants substances that are broken apart are written on the left and the products new substances formed are written on the right. For example this is an unbalanced equation. The head of the arrow typically points toward the right or the product side of the equation although some equations may indicate equilibrium with the reaction proceeding in both directions simultaneously.
We start by finding the stoichiometric ratio of the reactants. Ca 2 2Cl 2Ag 2NO 3 Ca 2 2NO 3 2AgCl Comparing the reactants and the products of the ionic equation and the chemical equation it can be observed that the Ca 2 calcium ion and the NO 3 nitrate ions are present on both sides of the ionic equation. Reactants are the substances that enter inti a chemical reaction and the products are the substances formed.
The substances to the left of the arrow in a chemical equationare called reactants. The equation is read as 4 moles of ammonia require 5 moles of oxygen to produce 4 moles of nitric acid and 6 moles of water. In relation to my course teaching can change the lives of the students.
On the other hand a chemical equation is the symbolic representation of a chemical reaction. Gwendolyn Aceves _____ Chemical Equations and Reactions What is a Chemical Equation.
The seemingly obvious solution to a FireWire ouster is the one Apple released this fall. I wasnt aware of them until just now but Kanguru apparently makes one called FireFlash available up to 8 GB capacity.

The Tragedy Of Firewire Collaborative Tech Torpedoed By Corporations Ars Technica
Add-A-Port Cable Expansion CNX-187 Firewire Female USB Female x2 CB-ADD-xxx.

What is the pc equivalent of firewire. Thunderbolt is the latest technology for connecting external devices to your PC and is replacing Firewire 1394 as the standard connection for audio recording interfaces and pro video devices. The FA-66 is a great bit of kit with all the ins and outs and features that I need. FireWire is not unique to any OS.
Like USB FireWire is a plug-and-play connection. What is the firewire equivalent of lsusb and lspci. Just connect a device to your PC by using a FireWire cable run the cable from the device to the FireWire port on your PC and youre ready to roll.
Your only options are either a PCI-E card that goes inside the machine not sure if a NUC can take expansion cards though with Firewire ports on it a machine with native Firewire or a laptop with an older ExpressCard slot or indeed in the really old days a PCMCIA slot and a Firewire card that fits. For example when I plug in the firewire connection to my Seagate Free Agent XTreme external 1TB. Always Wiki before you call.
Since then its been updated to FireWire 800. My ten year old or so desktop non-Mac PC that runs Windows 10 has FireWire ports that I use to connect of a Nikon film scanner. Fast Data transfer rates of 100 200 400 and 800 Mbits today and soon 1600 and 3200 Mbitss over copper wire and or fiber optic cable equivalent to 10 20 40.
This one-way adapter you cant use it to convert a Mac FireWire. It works with a variety of. Cleaning - Unplug the 8pre from the computer and clean only with a dry cloth.
Ventilation - Do not block any ventilation openings. Install in accordance with the manufacturers instructions. Index of s5r6linux1394ls1394 However I note it does not appear to give the Vendor-IDDevice-ID or if it does I could not figure it out.
Pentium or equivalent computer with one available PCI slot. Ive tried these and combinations including downloading the firewire lagacy driver but to no avail. You just install a FireWire card in your computer if it doesnt have it on the mainboard already.
MSI Global English Forum - Index. Is there a firewire equivalent of a USB memory stick. What is USB Firewire.
Apple first included FireWire in some of its 1999 Macintosh models and most Apple Macintosh computers manufactured. Hi Gary Theres no way to convert USB to Firewire as the two are such different protocols. IEEE 1394 is an interface standard for a serial bus for high-speed communications and isochronous real-time data transfer.
NN-400012-S8 Key Features and Benefits Three external IEEE 1394 FireWire ports support DV camcorders hard disk removable drives. SIIGs FireWire 800 3-Port PCI easily adds three external FireWire 800 1394b ports to your desktop computer. Note that these MUST BE 6-pin connectors as the 4-pin variant does not provide even a shred of power and thus the memory controller could not run.
It was developed in the late 1980s and early 1990s by Apple in cooperation with a number of companies primarily Sony and PanasonicApple called the interface FireWireIt is also known by the brands iLINK Sony and Lynx Texas Instruments. Its 29 Thunderbolt to FireWire Adapter. If the computer supports Thunderbolt 3 this will typically be a main feature listed in the manual and on the product page for the computer on the manufacturers website.
FireWire 800 is available on the non-Retina MacBook Pro Mac mini and Mac Pro. The FireWire 800 3-Port PCI delivers serial bus data transfer rates up to 800Mbs supports both asynchronous and isochronous data transfer modes and allows you to hot-swap devices connect and detach without first turning your system off. FireWire 3-Port PCI 3-port 1394 FireWire PCI adapter Part.
Real-time bi-directional multi-speed data transfer for all compliant applications read as co-processor and multi-processor ready. Check your USB-C port Some USB-C ports such as the ones pictured below have a Thunderbolt logo printed next to them to identify the ports as Thunderbolt 3 compatible. I installed the script under homeoldcpubin.
ASUS Mother Board P4S533-VX. It was developed in the late 1980s and early 1990s by Apple who called it FireWireThe 1394 interface is comparable to USB though USB has more market share. Lets say you have your digital camcorder connected to your home computer.
IEEE 1394 is an interface standard for a serial bus for high-speed communications and isochronous real-time data transfer. Sony Desktop Computer Vaio PVC-7762 or equivalent. The best I could find was ls1394 with script here.
The firewire card is a firewire 400 with TI chipset. The PC equivalent to FireWire is get this FireWire. FireWire at WikipediaFireWire at a glance.
Heat - Do not install the 8pre near any heat sources such as radiators heat registers stoves or another. When your computer powers up it queries all of the devices connected to the bus and assigns each one an address a process called enumerationFireWire is plug-and-play so if you connect a new FireWire device to your computer the operating system auto-detects it and asks for the driver disc. A FireWire port can support 63 devices using a daisy-chaining technique.
Do not use liquid or aerosol cleaners. It was originally developed by Apple and released in 1999as FireWire 400 when the very first PowerMac G3 was launched. To replace it with an equivalent USB model would cost quite a bit and as I only dabble it doesnt seem viable.
If the difference between the electronegativities of the two atoms is small neither atom can take the shared. A polar covalent bond is a covalent bond in which the atoms have an unequal attraction for electrons and so the sharing is unequal.

Smells Unit Investigation Iv Ppt Download
The greater the difference between atom electronegativity values the more polar the chemical bond formed between them.
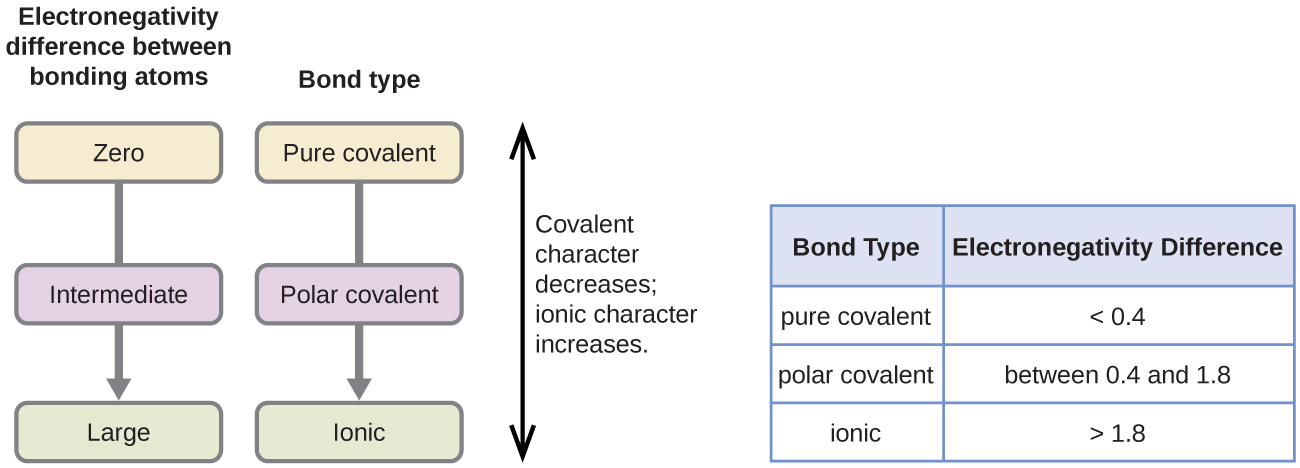
What does electronegativity have to do with polarity. The difference in electronegativity between two atoms determines how polar a bond will be. Think of it as a custody arrangement. When two atoms form a covalent bond the electronegativity values of the atoms involved in forming the bond dictate the polarity of the bond formed.
The most electronegative element is fluorine. A bond in which the electronegativity difference between the atoms is between 04 and 17 is called a polar covalent bond. If we have a covalent bond whose.
So higher electronegativity helps atoms take more control over shared electrons creating partial negative regions and partial positive regions which result in dipoles that cause polarity. Some textbooks or web sites use 17 Obviously there is a wide range in bond polarity with the difference in a C-Cl bond being 05 -- considered just barely polar -- to the difference the H-O bonds in water being 14 and in H-F the. Electronegativity is the tendency of an atom to attract.
In polar covalent bonds the electrons are shared unequally as one atom exerts a stronger force of attraction on the electrons than the other. Electronegativity is defined as the ability of an atom in a particular molecule to attract electrons to itself the greater the value the greater the attractiveness for electrons. The electron density that comprises the covalent bond is located halfway between the two atoms.
Electronegativity of elements increases across the period and decreases down the group. Electronegativity is an atoms tendency to attract electrons to itself in a chemical bond. Electronegativity and Polarity A critical property of molecules that depends on electronegativity is bond polarity.
The more electronegative element will attract electron density towards itself resulting in uneven charge distribution. Electronegativity is an elements attraction towards electrons in a covalent bond. In a polar covalent bond sometimes simply called a polar bond the distribution of electrons around the molecule is no longer symmetrical.
A quantity termed electronegativity is used to determine whether a given bond will be nonpolar covalent polar covalent or ionic. Therefore these two terms are closely related terms. Atoms of different elements have a different number of protons for which different attracting powers.
When a chlorine atom covalently bonds to another chlorine atom the shared electron pair is shared equally. Electronegativity is the measure of the power of an atom to pull the bond pair towards itself when two atoms are involved in a covalent bond. The typical rule is that bonds with an electronegativity difference less than 16 are considered polar.
Electronegativity and Polar Covalent Bonding Electronegativity is the strength an atom has to attract a bonding pair of electrons to itself. The higher the electronegativity is the more it attracts the electrons towards it. Electronegativity is how strongly an atoms attracts electrons - atoms with high electronegativity eg F have electron spending more time closer to them within a bond and do making.
The ability of an atom to attract a pair of electrons in a chemical bond is called its electronegativity. Check the electronegitivityThe polarity of a bond is determined by the differences in electronegativity of the reacting elements. 5K views View 8 Upvoters.
When two atoms combine the difference between their electronegativities is an indication of the type of bond that will form. Electronegativity is a measure of the ability of an atom to attract a pair of electrons to itself in a covalent bond. If the electronegativity difference is over4 then generally that means that its polar.
The ability of an atom in a molecule to attract shared electrons is called electronegativity. Polar bonds result from that uneven sharing of those electrons. Polarity arises due to the differences in electronegativity.
Bonds formed can be polar or non-polar. The key difference between electronegativity and polarity is that electronegativity is the tendency of an atom to attract the electrons in a bond towards it whereas polarity means the separation of the charges. The least electronegative or most electropositive element is francium.
A polar bond is a bond between two atoms of varying electronegativity. Electronegativity is the measure of the ability of an atom to attract electrons to itself. Electronegativity is affected by the atomic number and the distance between the valence electrons and its nucleus.
Electronegativity Definition Electronegativity is a chemical property that measures the tendency of an atom to attract electrons towards itself. Basically if a molecule is polar it means that one side of the molecule has a higher affinity for electrons and will attract them more strongly leading to a more positively charged side and a more negatively charged side.
Types of Chemical Reactions. The representation of a chemical reaction in the form of symbols substances is known as chemical equation.
Chemical Reactions And Equations Ck 12 Foundation
An arrow points from the reactant to the products.
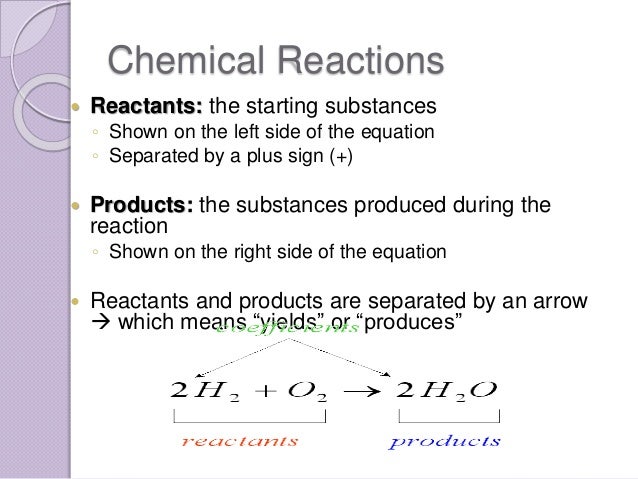
What are the reactants in a chemical equation. For example iron Fe and sulfur S combine to form iron sulfide FeS. 2Mg O 2 2MgO. The equation in which number of atoms of all the molecules is equal on both sides of the equation is known as balanced chemical equation.
A chemical equation consists of the chemical formulas of the reactants the starting substances and the chemical formula of the products substances formed in the chemical reaction. Chemical reactions can be written in chemical equation form which should always be balanced. There do not have to be multiple reactants in a reaction however.
Carbon Dioxide CO2 Water H2 ATP Energy This equation means that plants take C02 carbon dioxide and water and with the assistance of solar energy turn it into glucose and oxygen O2. Fe s S s FeS s The plus sign indicates that iron reacts with sulfur. They indicate the number of each chemical species that reacts or is formed.
Methane and oxygen oxygen is a diatomic two-atom element are the reactants while carbon dioxide and water are the products. The two are separated by an arrow symbol usually read as yields and each individual substances chemical formula is separated from others by a plus sign. A general reaction may be given by the equation.
The equation identifies the reactants starting materials and products resulting substances the formulas of the participants the phases of the participants solid liquid gas the direction of the chemical reaction and the amount of each substance. The arrow signifies that the reaction forms or yields iron sulfide the product. A B C.
The reactants of cellular respiration are. In a decomposition reaction such as. Reactants are converted to products and the process is symbolized by a chemical equation.
The chemical equation for this reaction is written as. The head of the arrow typically points toward the right or the product side of the equation although some equations may indicate equilibrium with the reaction proceeding in both directions simultaneously. The reactants are all written on the left-hand side of the equation with the products on the right-hand side.
A chemical equation consists of reactants products and an arrow showing the direction of reaction. 411 2 H 2 O 2 2 H 2 O Chemical formulas and other symbols are used to indicate the starting materials or reactants which by convention are written on the left side of the equation and the final compounds or products which are written on the right. Formation of chemical reaction.
All the reactants and products are gases indicated by the gs in parentheses. The arrow is read as the word yields. 311 2 H 2 O 2 2 H 2 O Chemical formulas and other symbols are used to indicate the starting materials or reactants which by convention are written on the left side of the equation and the final compounds or products which are written on the right.
A chemical equation expresses the net change in composition associated with a chemical reaction by showing the number of moles of reactants and products. The number of atoms of the reactants and products need to be balanced. A chemical equation describes what happens in a chemical reaction.
In the symbolic equation chemical formulas are used instead of chemical names for reactants and products while symbols are used to indicate the phase of each substance. In the equation above the zinc and sulfur are the reactants that chemically combine to form the zinc sulfide product. In chemical reactions atoms are never created or destroyed.
They consist of chemical or structural formulas of the reactants on the left and those of the products on the right. A chemical equation shows the chemical formulas of substances that are reacting and the substances that are produced. There is a standard way of writing chemical equations.
It should be apparent that the chemical shorthand method is the quickest and clearest method for writing chemical equations. In a complete chemical equation the two sides of the equation must be present on the reactant and the product sides of the equation. Glucose sugar Oxygen O2 The products of cellular respiration are.
They are separated by an arrow which indicates the direction and type of the reaction. A single reactant breaks down to yield two or more products. The reactants for photosynthesis are light energy water carbon dioxide and chlorophyll while the products are glucose sugar oxygen and water.
C A B. Chemical equations are balanced for mass and charge meaning the number and type of atoms on the left side of the arrow is the same as the number of type of atoms on the. In this example A and B are the reactants and C is the product.
A chemical equation is written with the reactants on the left side of an arrow and the products of the chemical reaction on the right. A single product is formed from two or more reactants. The same atoms that were present in the reactants are present in the productsthey are merely reorganized into different arrangements.
But because each component has its own molar mass equations also implicitly define the way in which the masses of products and reactants are related. Chemical equations are used to graphically illustrate chemical reactions. C is the reactant while A and B are the products.
The greater the difference between atom electronegativity values the more polar the chemical bond formed between them. A polar covalent bond is a covalent bond in which the atoms have an unequal attraction for electrons and so the sharing is unequal.

Electronegativity And Polarity Ocr A Level Teaching Resources
The absolute values of the electronegativity differences between the atoms in the bonds HH HCl and NaCl are 0 nonpolar 09 polar covalent and 21 ionic respectively.

What electronegativity is polar. Fluorine with the highest electronegativity of 4 is the most non-metallic element and caesium with the lowest electronegativity of 07 is the most metallic element. A bond in which the electronegativity difference between the atoms is between 05 and 20 is called a polar covalent bond. In a chemical bond between two atoms the bond is said to be polar if the two atoms have a difference in their electronegativity.
Electronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons while polarity is the property of having poles or being polar. What you should do is look only at the two atoms in a given bond. An atoms electronegativity is affected by both its atomic number and the distance at which its valence electrons reside from the charged nucleus.
The least electronegative or most electropositive element is francium. Oxygen difluoride is also known by its other name hypofluorous anhydride. If the difference in electronegativity is greater than 17 the character of the bond will be ionic.
Electronegativity χ was defined as the ability of an atom in a molecule or an ion to attract electrons to itself. Only the absolute difference is important. When the difference is very small or zero the bond is covalent and nonpolar.
In a polar covalent bond sometimes simply called a polar bond the distribution of electrons around the molecule is no longer symmetrical. Fluorine the most electronegative element is assigned a value of 40 and values range down to caesium and francium which are the least electronegative at 07. If the difference in electronegativity is between 04 and 17 the character of the bond is polar covalent.
Thus there is a direct correlation between electronegativity and bond polarity. Electronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons. The Pauling scale is the most commonly used.
Electronegativity symbol χ measures the tendency of an atom to attract a shared pair of electrons or electron density. Greater the difference of electronegativity of two atoms more is the polarity of that bond. According to electronegativity the bond is ionic if the difference between the electronegativity of atoms is greater than 17.
A compound becomes Polar when an electronegativity difference is found between the bonded atoms. In a polar covalent bond sometimes simply called a polar bond the distribution of electrons around the molecule is no longer symmetrical. A molecule will be nonpolar if-If the arrangement is symmetrical canceling the polar bonds will lead to a nonpolar molecule.
Lets have an idea about the factors which make a molecule polar or nonpolar. When it is large the bond is polar covalent or ionic. A polar covalent bond is a covalent bond in which the atoms have an unequal attraction for electrons and so the sharing is unequal.
Electronegativity values are useful in determining if a bond is to be classified as nonpolar covalent polar covalent or ionic. When a chlorine atom covalently bonds to another chlorine atom the shared electron pair is shared equally. Explanatory video and quiz on electronegativity values on the Pauling scale and how it changes down the group and along the period Identification of most electronegative and least electronegative element.
The higher the associated electronegativity the more an atom or a substituent group attracts electrons. The degree to which an atom attracts electrons in a chemical bond is described by electronegativity. The most electronegative element is fluorine.
If you want to determine the polarity of a molecule then there are few points that must be kept in mind in order to find out whether a given molecule is polar or not. As a result the dipole moment of the molecule turns out to be nonzero making the OF2 a polar molecule. As the electronegativity increases the non-metallic character increases.
Firstly a molecule is polar or nonpolar depending on electronegativity structure and dipole moment. So the key difference between electronegativity and polarity is that the electronegativity is the tendency of an atom to attract the electrons in a bond towards it whereas polarity is the separation of the charges. As the electronegativity decreases the metallic character increases.
Greater the value of electronegativity of an atom more is its polarity. Electronegativity is an atoms tendency to attract electrons to itself in a chemical bond. Polarity is the electric charge distribution around atoms molecules or chemical groups.
The electronegativity of an atom is its strength with which it attracts the bonded pair to its side. Electronegativity and Polar Covalent Bonding Electronegativity is the strength an atom has to attract a bonding pair of electrons to itself. OF2 Oxygen difluoride is polar in nature because of its bent shaped geometrical structure and difference between the electronegativity of Oxygen and Fluorine atoms.
Calculate the difference between their electronegativity values. Based on difference in the electronegativity identification of polar nonpolar and ionic bond alongwith practice problems. The absolute values of the electronegativity differences between the atoms in the bonds HH HCl and NaCl are 0 nonpolar 09 polar covalent and 21 ionic respectively.
A bond in which the electronegativity difference between the atoms is between 05 and 21 is called a polar covalent bond. When the difference is very small or zero the bond is covalent and nonpolar. The bond is polar covalent if the.
2 Polar and Non-polar Bonds. When it is large the bond is polar covalent or ionic. The electron density that comprises the covalent bond is located halfway between the two atoms.
The polarity of a bondthe extent to which it is polaris determined largely by the relative electronegativities of the bonded atoms.
