When one or more reactants are converting to products they may go through different modifications and energy changes. The law of conservation of matter has been satisfied.
The products are carbon dioxide gas and water vapor.

What are reactants and products in a chemical equation. Reactants are substances that start a chemical reaction. A chemical equation is written with the reactants on the left side of an arrow and the products of the chemical reaction on the right. A chemical reaction results in the forming or breaking apart of chemical bonds between various elements.
The products are carbon dioxide gas and water vapor. When a candle burns the reactants are fuel the candlewick and wax and oxygen in the air. The head of the arrow typically points toward the right or the product side of the equation although some equations may indicate equilibrium with the reaction proceeding in both directions simultaneously.
2 SO 2 g O 2 g Reactants 2 SO 3 g Products. Reactants and products are the two major components of a chemical reaction. In a chemical formula the reactants are on the left side of the arrow and the products are on the right.
A product is a substance that is present at the end of a chemical reaction. On the flip side the products are present on the left side of the equation. The chemical equation for this reaction is written as.
Products are substances that are produced in the reaction. Stoichiometry ˌ s t ɔɪ k i ˈ ɒ m ɪ t r i is the calculation of reactants and products in chemical reactions in chemistry. The reactants are consumed throughout the chemical reaction while products are not consumed or used up in the reaction.
Reactants and products A chemical equation shows the reactants the starting substances on the left side of the arrow and the products the substances formed during the reaction on the right side of the arrow. The substances at the beginning are known as reactants and the substances after the reaction are known as products. When a candle burns the reactants are fuel the candlewick and wax and oxygen in the air.
You cant change one element into another in a chemical reaction that happens in nuclear reactions. A chemical equation consists of reactants products and an arrow showing the direction of reaction. Reactants are substances that start a chemical reaction.
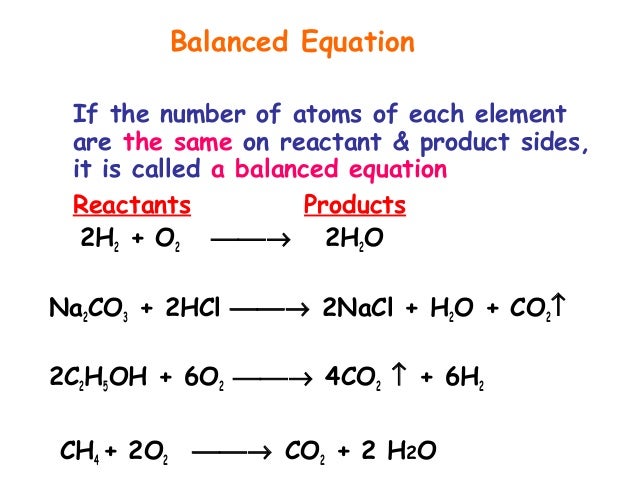
When the reactants and products of a chemical equation have the same number of atoms of all elements present we say that an equation is balanced. Reactants vs Products A reaction is a process of converting a set of substances into another set of substances. There are two oxygen atoms in the reactants and two atoms of oxygen in the product.
There are various types of chemical reactions such as acid-base reactions redox reactions and combustion reactions. Stoichiometry is founded on the law of conservation of mass where the total mass of the reactants equals the total mass of the products leading to the insight that the relations among quantities of reactants and products typically form a ratio of positive integers. The reactants in the chemical reaction are usually present on the right side of the arrow.
This chemistry video tutorial explains the process of predicting the products of chemical reactions. The reactants for photosynthesis are light energy water carbon dioxide and chlorophyll while the products are glucose sugar oxygen and water. The arrow can be read as yields or react to form.
Potassium hydroxide sulfuric acid potassium sulfate water Chemical. There is a standard way of writing chemical equations. Products are substances that are produced in the reaction.
These reactions can result in many different outcomes which can be affected by temperature pressure and the amount of the reactants. The substance s to the right of the arrow are called products. 311 2 H 2 O 2 2 H 2 O Chemical formulas and other symbols are used to indicate the starting materials or reactants which by convention are written on the left side of the equation and the final compounds or products which are written on the right.
Sulfur dioxide and oxygen SO 2 O 2 are reactants and sulfur trioxide SO 3 is the product. Reactants and Products in Chemical Reactions In a chemical reaction substances elements andor compounds called reactants are changed into other substances compounds andor elements called products. Products are the chemical species that can be found after the completion of the reaction.
Writing Chemical Equations When sulfur dioxide is added to oxygen sulfur trioxide is produced. This video contains plenty of examples and practice pro. In the equation above the zinc and sulfur are the reactants that chemically combine to form the zinc sulfide product.
The equation in which number of atoms of all the molecules is equal on both sides of the equation is known as balanced chemical equation. The reactants are potassium hydroxide and sulfuric acid the products are potassium sulfate and water the word equation is. Reactants are the starting material of a chemical reaction.
The representation of a chemical reaction in the form of symbols substances is known as chemical equation. All proper chemical equations are balanced.

Writing And Balancing Chemical Equations Introductory Chemistry Lecture Lab
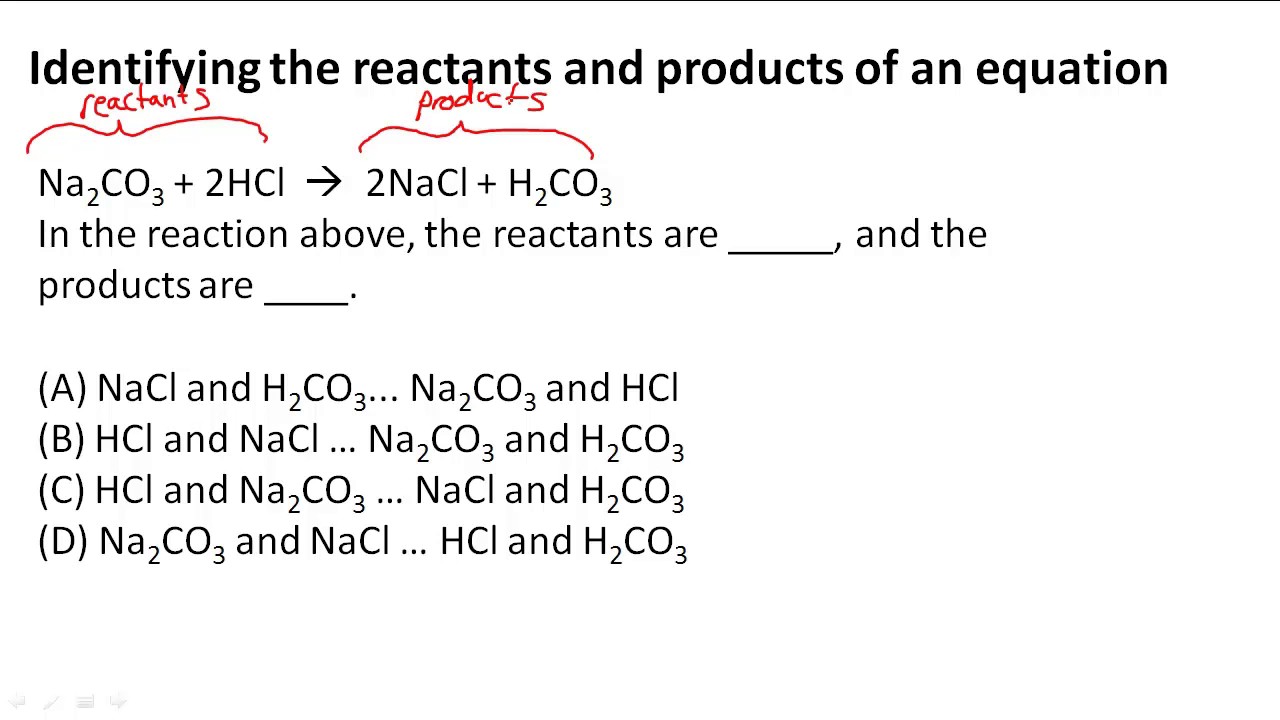
Identifying The Reactants And Products Of An Equation Youtube
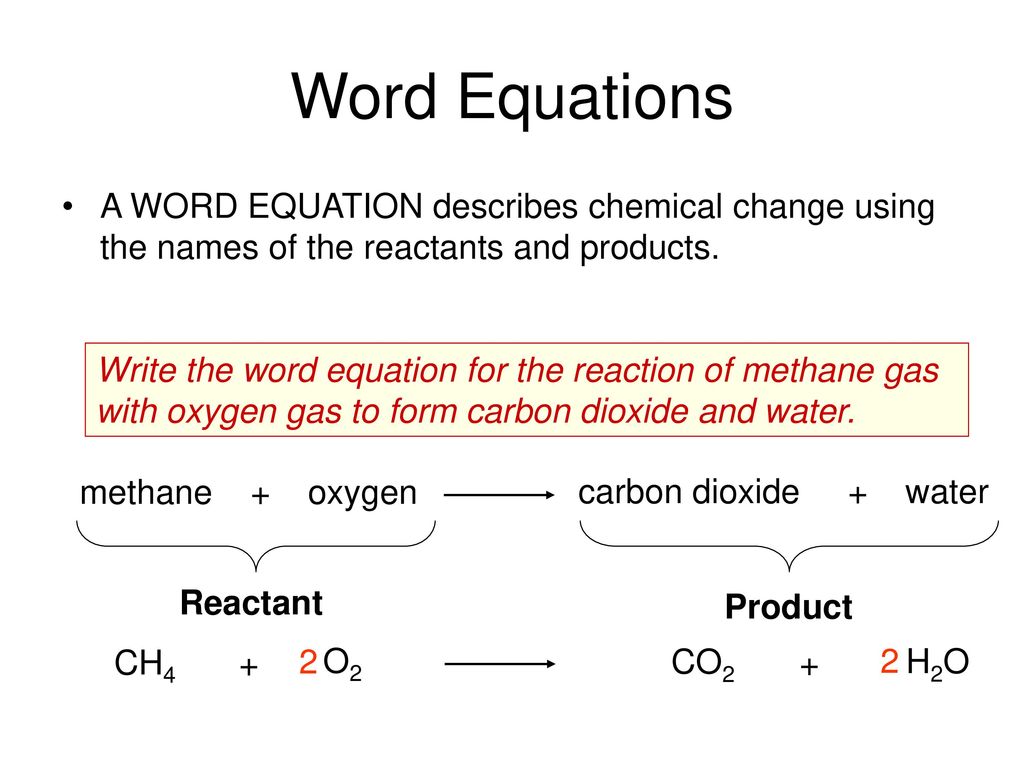
Chemical Equations Depict The Kind Of Reactants And Products Ppt Download
Chem 101 General Chemistry Topic