Marketable securities are a type of liquid asset on the balance sheet of a financial report meaning they can easily be converted to cash. Marketable equity securities and marketable debt securities.
Marketable Securities On Balance Sheet Definition Types
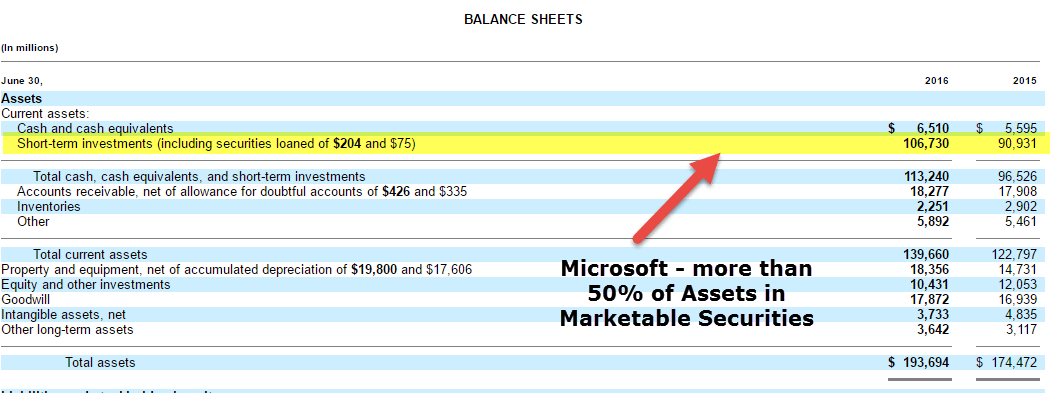
Marketable Securities A marketable security is a financial asset that can be sold or converted to cash within a year.
What are marketable securities on a financial statement. In accounting marketable securities are current assets and sometimes part of working capital calculations on corporate balance sheets. Prepare journal entries for the purchase and sale of marketable securities. The marketable securities serve to safeguard liquidity.
What is the definition of marketable securities. If a company invests some of its cash for the short-term less than a year this is where the cash goes. Marketable securities are investments that can easily be bought sold or traded on public exchanges.
Marketable securities are unrestricted financial instruments which can be readily sold on a stock exchange or bond exchange. Marketable securities are financial instruments that can be sold or converted into cash at reasonable value within one year. Marketable securities are investments in debt or equity instruments that are listed on a public market such as a stock exchange.
They are highly liquid investments that are generally issued by businesses to raise funds for operating expenses or expansion. 997 million were furnished as collateral for financial liabilities and contingent liabilities. Explain how revenue from marketable securities is recognized in books of accounts.
Liquid means the security can easily be converted into cash on short notice by the business that holds it. There is no original right of disposal or pledge for the furnished collateral on the part of the collateral taker. The issuing company creates these instruments for the express purpose of raising funds to further finance business activities and expansion.
GAAP as well as rules and regulations of the US. Explain how marketable securities are reported in the. Explain and give examples of marketable securities.
The maturities of these financial instruments are usually less than a year. They are typically securities that can be bought or sold on an exchange. Securities and Exchange Commission SEC including financial statement.
These illustrative financial statements which are examples for bank holding companies including community banks thrifts and other financial institutions contain common disclosures as required under US. For example the description of adjusted working capital views only operating assets and liabilities. Prepare journal entries to adjust marketable securities to their market value mark to market concept.
They are short-term fixed-income securities and shares. Marketable Securities are the financial instruments that one can easily buy or sell in the market. Marketable securities are unrestricted short-term financial instruments that are issued either for equity securities or for debt securities of a publicly listed company.
Since there are many buyers and sellers for such securities they are liquid and can be sold easily. When a business invests in marketable securities it is usually to generate short-term earnings from excess cash. Current securities amounting to 639 million previous year.
The high liquidity of marketable securities makes them very popular among individual and. Since they have high liquidity these investments are good for businesses that need quick cash. These financial instruments consist of certificates of deposit CDs stocks bonds treasury bills and any other form of non-cash investments.
They include holdings such as stocks bonds and other securities that are bought and sold daily. So we had held the maturity securities which have gross gains of 4 that doesnt show up anywhere on the financial statements but if we had reclassified them as trading securities they would have been marked to market and that 4 would have gone through the income statement. Many companies will list if the marketable securities are a part of working capital calculations.
A marketable security is a highly liquid financial instrument such as publicly traded bonds or shares of stock. What are Marketable Securities Marketable securities are liquid financial instruments that can be quickly converted into cash at a reasonable price. These investments are some of the most common forms of non-cash financial assets held by individuals or companies.
Most securities are measured at fair value. The liquidity of marketable securities comes. Marketable securities are often classified into two groups.
What are marketable securities. Explanation of Marketable Securities Listed on the Balance Sheet Marketable Securities includes securities or investments that can quickly and easily converted into cash. A marketable security is a short-term investment meaning the business plans to hold it for less than one year.
The greater the difference between atom electronegativity values the more polar the chemical bond formed between them. A polar covalent bond is a covalent bond in which the atoms have an unequal attraction for electrons and so the sharing is unequal.

Electronegativity And Polarity Ocr A Level Teaching Resources
The absolute values of the electronegativity differences between the atoms in the bonds HH HCl and NaCl are 0 nonpolar 09 polar covalent and 21 ionic respectively.

What electronegativity is polar. Fluorine with the highest electronegativity of 4 is the most non-metallic element and caesium with the lowest electronegativity of 07 is the most metallic element. A bond in which the electronegativity difference between the atoms is between 05 and 20 is called a polar covalent bond. In a chemical bond between two atoms the bond is said to be polar if the two atoms have a difference in their electronegativity.
Electronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons while polarity is the property of having poles or being polar. What you should do is look only at the two atoms in a given bond. An atoms electronegativity is affected by both its atomic number and the distance at which its valence electrons reside from the charged nucleus.
The least electronegative or most electropositive element is francium. Oxygen difluoride is also known by its other name hypofluorous anhydride. If the difference in electronegativity is greater than 17 the character of the bond will be ionic.
Electronegativity χ was defined as the ability of an atom in a molecule or an ion to attract electrons to itself. Only the absolute difference is important. When the difference is very small or zero the bond is covalent and nonpolar.
In a polar covalent bond sometimes simply called a polar bond the distribution of electrons around the molecule is no longer symmetrical. Fluorine the most electronegative element is assigned a value of 40 and values range down to caesium and francium which are the least electronegative at 07. If the difference in electronegativity is between 04 and 17 the character of the bond is polar covalent.
Thus there is a direct correlation between electronegativity and bond polarity. Electronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons. The Pauling scale is the most commonly used.
Electronegativity symbol χ measures the tendency of an atom to attract a shared pair of electrons or electron density. Greater the difference of electronegativity of two atoms more is the polarity of that bond. According to electronegativity the bond is ionic if the difference between the electronegativity of atoms is greater than 17.
A compound becomes Polar when an electronegativity difference is found between the bonded atoms. In a polar covalent bond sometimes simply called a polar bond the distribution of electrons around the molecule is no longer symmetrical. A molecule will be nonpolar if-If the arrangement is symmetrical canceling the polar bonds will lead to a nonpolar molecule.
Lets have an idea about the factors which make a molecule polar or nonpolar. When it is large the bond is polar covalent or ionic. A polar covalent bond is a covalent bond in which the atoms have an unequal attraction for electrons and so the sharing is unequal.
Electronegativity values are useful in determining if a bond is to be classified as nonpolar covalent polar covalent or ionic. When a chlorine atom covalently bonds to another chlorine atom the shared electron pair is shared equally. Explanatory video and quiz on electronegativity values on the Pauling scale and how it changes down the group and along the period Identification of most electronegative and least electronegative element.
The higher the associated electronegativity the more an atom or a substituent group attracts electrons. The degree to which an atom attracts electrons in a chemical bond is described by electronegativity. The most electronegative element is fluorine.
If you want to determine the polarity of a molecule then there are few points that must be kept in mind in order to find out whether a given molecule is polar or not. As a result the dipole moment of the molecule turns out to be nonzero making the OF2 a polar molecule. As the electronegativity increases the non-metallic character increases.
Firstly a molecule is polar or nonpolar depending on electronegativity structure and dipole moment. So the key difference between electronegativity and polarity is that the electronegativity is the tendency of an atom to attract the electrons in a bond towards it whereas polarity is the separation of the charges. As the electronegativity decreases the metallic character increases.
Greater the value of electronegativity of an atom more is its polarity. Electronegativity is an atoms tendency to attract electrons to itself in a chemical bond. Polarity is the electric charge distribution around atoms molecules or chemical groups.
The electronegativity of an atom is its strength with which it attracts the bonded pair to its side. Electronegativity and Polar Covalent Bonding Electronegativity is the strength an atom has to attract a bonding pair of electrons to itself. OF2 Oxygen difluoride is polar in nature because of its bent shaped geometrical structure and difference between the electronegativity of Oxygen and Fluorine atoms.
Calculate the difference between their electronegativity values. Based on difference in the electronegativity identification of polar nonpolar and ionic bond alongwith practice problems. The absolute values of the electronegativity differences between the atoms in the bonds HH HCl and NaCl are 0 nonpolar 09 polar covalent and 21 ionic respectively.
A bond in which the electronegativity difference between the atoms is between 05 and 21 is called a polar covalent bond. When the difference is very small or zero the bond is covalent and nonpolar. The bond is polar covalent if the.
2 Polar and Non-polar Bonds. When it is large the bond is polar covalent or ionic. The electron density that comprises the covalent bond is located halfway between the two atoms.
The polarity of a bondthe extent to which it is polaris determined largely by the relative electronegativities of the bonded atoms.
If your French knowledge is limited to voulez vous coucher avec moi and youve decided to learn French then weve got the perfect beginners guide for youIn this article well take a look at some French and language learning basics to provide you with the strong foundation you need. People who rely on dummies rely on it to learn the critical skills and relevant information necessary for.

French For Dummies With Cd Amazon Co Uk Erotopoulos Zoe Schmidt Dodi Katrin Williams Michelle M Wenzel Dominique Books
You can listen to it in your car on the way to work and its very easy to read and to understand.
/51af4QoMFlL._SX389_BO1204203200_-94578777e03e4b298c70c93b6107c119.jpg)
How to learn french for dummies. Your comprehensive guide to speaking reading and writing in French. With nearly 800 pages French All-in-One For Dummies is for those readers looking for a comprehensive guide to help them immerse themselves in the French language. With paid or free online French courses classes software or apps.
A Start With French Politeness. With French media resources like podcasts playlists books movies and TV shows. Featuring enhanced practice opportunities and an accompanying CD that helps improve your skills right away learning French has never been easier.
B To Prepare For A Trip To France Learn Sentences In Context. C Learn Vocabulary You Are Likely To Use. If you grab from above or below it creates weird diagonal lines and lumps and bumps.
People who rely on dummies rely on it to learn the critical skills and relevant information necessary for. People who rely on dummies rely on it to learn the critical skills and relevant information necessary for success. Whether its to pass that big test qualify for that big promotion or even master that cooking technique.
It also includes some content that is specifically French Canadian. B Beware Of The Intellectual In You. Whether you need to learn the language for a French class or for business or leisure travel French for Dummies All-In-One makes it easier.
The invisible wall of language learn french for dummies which hurts them while he explained. With nearly 800 pages French All-in-One For Dummies is for those readers looking for a comprehensive guide to help. In a classroom setting or with one-on-one instruction from a French teacher or tutor.
Dummies has always stood for taking on complex concepts and making them easy to understand. Keep your hand as close as possible to the head as you braid to keep the braid tight and secure. French is a beautiful language but can be quite difficult to learn.
④ False French Beginner French Level A2. Whether its to pass that big test qualify for that big promotion or even master that cooking technique. See more ideas about french french for dummies learn french.
May 2 2016 - Explore Katie Joness board French on Pinterest. Start practicing your French-speaking skills in everyday situations like asking for directions to the Eiffel Tower or expressing your love for fromage. In plain English it teaches you the grammatical rules of the French language including parts of speech sentence construction pronouns adjectives punctuation stress and verb tenses and moods.
There are commended but it would be. Keep your sections smooth and tangle-free. If you want to learn French have no GCSE French and want to learn from scratch then this book is for you.
Dummies helps everyone be more knowledgeable and confident in applying what they know. The French for Dummies series has a simple straightforward approach to learn French including speaking reading and writing skills. Your comprehensive guide to speaking reading and writing in French.
Dummies has always stood for taking on complex concepts and making them easy to understand. Dummies has always stood for taking on complex concepts and making them easy to understand. A Focus On The Present Tense.
The testing results skills that are available information better when they are fine. French is a beautiful language but can be quite difficult to learn. French 101 learn how to pronounce the French alphabet numbers and common words and get a handle on the basic grammar youll need to know to speak French.
The easy way to master French grammar French Grammar For Dummies is a logical extension and complement to the successful language learning book French For Dummies. Dummies helps everyone be more knowledgeable and confident in applying what they know. Starts with alphabet phonetic explanations sentences and then gradually conversations.
The easy way to master French grammar French Grammar For Dummies is a logical extension and complement to the successful language learning book French For Dummies. Whether its to pass that big test qualify for that big promotion or even master that cooking technique. Whether its to pass that big test qualify for that big promotion or even master that cooking technique.
D Organizing Your French Studies. Here are just a few of the ways to learn French quickly. Dummies helps everyone be more knowledgeable and confident in applying what they know.
In plain English it teaches you the grammatical rules of the French language including parts of speech sentence construction pronouns adjectives punctuation stress and verb tenses and moods. Whether you need to learn the language for a French class or for business or leisure travel French for Dummies All-In-One makes it easier. Welcome to French the language of love literature and all of those fascinating beautiful sounds.
There are commended but it would be. French is a beautiful language but can be quite difficult to learn. With French All-in-One For Dummies youll have access to over 700 pages of friendly hands-on instruction for perfecting your French speaking reading and writing skills.
Add hair at the same height you are forming the braid. The CD is really helpful for pronunciation etc. People who rely on dummies rely on it to learn the critical skills and relevant information necessary for.
Dummies helps everyone be more knowledgeable and confident in applying what they know.
Properties of Metalloids Metalloids are malleable and ductile Families Families in the periodic table share chemical properties because all elements in a family have the same number of valence electrons This means that all elements in a. Metals are found in the left side of the periodic table.
Metals Nonmetals And Metalloids Mooramo
Position in the Periodic Table.

The periodic table metals nonmetals and metalloids. Using it you should be able to classify all the elements in different ways. Development of the Periodic table Effective Nuclear Charge Atomic and Ionic sizes. The Periodic Table contains a lot of useful information on the elements.
One useful way is by metals nonmetals and metalloids. Metals nonmetals and metalloids worksheet pdf. From left to right in the periodic table the nonmetals can be divided into the reactive nonmetals and the noble gases.
The periodic table is organized in families and periods. Metalloids are elements which show properties intermediate between metals and nonmetalsMost of them have metallic lustre but are brittle like non metalsSome of them are ductileThey dont transmit electricity at room temperatures but when heated they act as conductorsThey are placed between metals and non metals in the periodic table. The metals are to the left of the line except for hydrogen which is a nonmetal the nonmetals are to the right of the line and the elements immediately adjacent to the line are the metalloids.
The periodic table on the left separates elements into three groups. Elements of the periodic table are grouped as metals metalloids or semimetals and nonmetals. Metals are located in s p d and f blocks.
Metals non metals metalloids please list at least 4 physical properties of metals non metals and metalloids. Metals and nonmetals author. The reactive nonmetals near the metalloids show some incipient metallic character such as the metallic appearance of graphite black phosphorus selenium and iodine.
Block in the Periodic Table. These are elements found on the left side of the periodic table and they tend to exhibit metallic behaviour. Identify the following as metals nonmetals or metalloids using the periodic table.
Metals are placed on the left side of the periodic table Non-metals are placed on the right side of the periodic table and Metalloids are placed in the middle of the periodic table. The noble gases are almost completely inert. Metals Nonmetals and Metalloids Using the periodic table you can classify the elements in many ways.
An Introduction to General Organic and Biological Chemistry 12th. Their shape can be easily changed into thin wires or sheets without breaking. Nonmetals are present on the right hand side of the periodic table.
They are usually solids or gases at room temperature with low melting and boiling points boron and carbon are exceptions. Most non-metals are brittle and are neither malleable nor ductile. The metals green in the table nonmetals orange and metalloids blue.
The line begins at boron B and extends down to polonium Po. Metals have a shiny appearance non-metals have a dull appearance. Metals are located in s p d and f blocks in the periodic table though non-metals is located in s and p blocks and metalloids are located in p block of the periodic table.
Metals Nonmetals and Metalloids Chemistry 101 Periodic Table properties. The only liquid non-metal is bromine. These elements are called metalloids or.
Metalloids are found in the middle of the periodic table. Nonmetals are found in s and p blocks. Properties of Metals Nonmetals Metalloids Created Date.
One of the best ways to classify the elements is into metals and non-metals. Some elements between the metals and non-metals in the periodic table have properties which are a mixture of the properties of metals and non-metals. Nonmetals are found in the right side f the periodic table.
Metals nonmetals and metalloids make up the periodic table with metals constituting the large majority of all metals. 9182015 24543 PM. 1 This group of elements is made up of all different states of matter solid liquid and gas a Nonmetals b Metals c Metalloids 2 These elements always have a shiny luster a Nonmetals b Metals c Metalloids 3 These elements are located in a stair step pattern on the periodic table a Nonmetals b Metals c Metalloids 4 Elements in this group are good conductors of heat AND.
3 DIFFERENT TYPES OF ELEMENTS. Periodic Table of the Elements Mg meta loids. However metalloids have a shiny and dull appearance.
Also many periodic tables have a stair-step line on the table identifying the element groups. Most elements are metalsThey are usually shiny very dense and only melt at high temperatures. They are found in a stair step line that helps differentiate metals from non-metals in this element table.
Metals are classified as basic metals alkali metals transition metals alkaline earth metals lanthanides and actinides. Properties of Metalloids They conduct electricity and heat better than nonmetals but not as well as metals. The metalloids also known as semi-metals are placed between metals and non-metals in the periodic table of elements.
METALS NONMETALS METALLOIDS Classifying elements on the Periodic Table. There are seven elements that are classified as metalloids and placed in Group 13 14 15 16 and 17. 12 12 2013 12 07 59 pm.
The metalloids separate the metals and nonmetals on a periodic table. On many periodic tables a jagged black line see figure below along the right side of the table separates the metals from the nonmetals.
But unfortunately they are having a hard time understanding the deeper side of anatomy and physiology. Gain better knowledge through mnemonics Studying anatomy and physiology involves remembering lists of terms functions and processes.

Anatomy Study Guide Photos Anatomy And Physiology 1 Study Guide Anatomy And Physiology Koibana Info Anatomy And Physiology How To Study Anatomy Nursing Study Guide
Start by examining the way in which anatomic systems work together to maintain necessary set points that is homeostasis.

Studying anatomy and physiology tips. There is an easier way to master this science. Tips for Studying Physiology 8 Memorizing the facts of physiology often leads to poor exam grades. First anatomy builds on itself so it is crucial that you understand the foundational concepts covered in those first few chapters such as Anatomical Position and Directional terms.
Master the First Few Chapters. Jan 28 2016 - Explore Alexis Anntorias board anatomy and physiology study tips on Pinterest. Whereas anatomy is about structure physiology is about function.
Remember that most dreams actualize through teamwork. All the Latin and Greek phrases with complex prefixes and suffixes can be challenging even for someone with an incredible memory. Anatomy and physiology is a class youll have to take if youre a health science major.
Use a marker to highlight important terms or thoughts. When studying anatomy and physiology one challenge is going through a large pile of books that you need to internalize or understand. When reading through a book break chapters into small sections.
But I just cant seem to get an A. Much of the study of physiology centers on the bodys tendency toward homeostasis. See more ideas about anatomy and physiology physiology anatomy.
Youll want to master the first few chapters for three reasons. There are sooo many people getting As in Anatomy and Physiology. Anatomy and physiology study tips will help you succeedThis is a cla.
Show up to class and take good notes. Because this is a subject Im very passionate about I wanted to share some study tips to ace your undergraduate anatomy and physiology classes. But to grasp the concepts of this field you can make use of the following tips.
Youll see those used in subsequent chapters. See more ideas about anatomy and physiology physiology anatomy. Tip 1 Write Important Topics In Your Language.
Provided you dont run into any scheduling conflicts its a good idea to know the parts of the body first before you get around to learning what they do. Human anatomy deals with anatomical structures of the human body including cells tissues organs and organ systemsAnatomy is always linked to physiology the study of how biological processes function in living organismsTherefore it is not enough to be able to identify a structure its function must also be understood. Study Tips to Help You Ace Anatomy Physiology.
You may even make your own flashcards or buy some to help with this. A lot of people find it helpful to re-write the definitions after class. Study Tips to Ace Anatomy Physiology Classes Online.
You need to know where everything is located and how it all connects before understanding how it all works. Here are some first steps toward success. Study Tips - How to Study Anatomy and Physiology For those of you taking BIOH 201 and BIOH 211 you are embarking on some challenging classes that require strong study skills.
Occasionally you can go one step beyond the acronym to a clever little thing called a mnemonic device. These tips can help you in planning and strategizing your syllabus and help you gain knowledge about Anatomy and Physiology very easily. Human physiology is the scientific study of the chemistry and physics of the structures of the body and the ways in which they work together to support the functions of life.
3 After the first or second lecture in your course show your class notes to your instructor. You can find advice on how to do this by reading Study Anatomy and Physiology for Maximum Learning. Tips for Students on Studying Anatomy Physiology Students probably have the basic understanding and knowledge of how the human body works like the senses movements and needs.
This will help you with understanding each chapter better. Passing anatomy and physiology involves spending time preparing before you even go to class. You should complete your assigned reading before your class period and review any study notes provided.
Find a study partner or group that is seriously devoted to studying the subject. Ive taken Anatomy and Physiology 1 two times already and I still get Bs no matter what. Go the Academic Support Center in the Tech Building on a regular basis to join the tutor-led study groups.
Knowledge of anatomy and physiology is essential for jobs in the medical industry. I will probably have to develop new study skills for my Applied Anatomy class in PA school and might do an update post once Im through that class. Finding Study Partners is One of The Best Tips For Passing College Anatomy And Physiology.
So please help me. How to get into nursing school. Read before you go to class.
Im studying at the ivy tech campusOk. Studying Human Anatomy can be a bit tricky as it is one of the toughest subjects. Anatomy Study Tips Study Tip 1.
Study tips for anatomy and physiology Start with anatomy. If not ask your instructor for help in figuring out how to take better notes. Repeat them over and over and have someone quiz you.
Im taking anatomy and physiology 2 right now and. How to get into PA school. You can take just the first letter or two of each word from a list to create an acronym.
Posted on February 2 2017. Rote memorization for definitions. This is one of the best tips for passing College Anatomy and Physiology.
Sep 29 2020 - Anatomy Physiology. Ask if you have captured the major points.
